
| Cr2O3 |
| ʵ�鷽�� | �й�ʵ����������ӷ���ʽ |
| ��G��β���Ⱥ�ֱ�ͨ���Լ���˳���� |



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
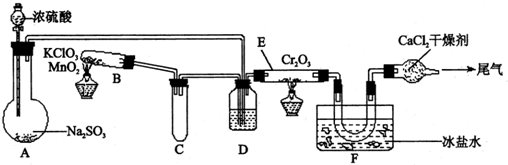
2(08�㽭ʡ������ѧģ��)ʵ�����и���2SO2��O2![]() 2SO3����H=-393.2 kJ?mol-1�������ͼ��ʾʵ��װ�����Ʊ�SO3���塣��ش��������⡣
2SO3����H=-393.2 kJ?mol-1�������ͼ��ʾʵ��װ�����Ʊ�SO3���塣��ش��������⡣
 |
��1��ʵ��ǰ��������еIJ����ǣ���������ƣ�����д������̣�������������
��2����Aװ���м���Na2SO3�����ͬʱ������Ӽ���ˮ��Ȼ���ٵμ�Ũ���ᡣ�Ӽ���ˮ�������� ��
��3��С�Թ�C��������
��4�����ƿD��ʢ���Լ��� ��װ��D������������ ������ ����
�� ��
��5��ʵ���е�Cr2O3�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȣ��Է��¶ȹ��ߣ���������ԭ���� �� ��
��6��װ��F��U�����ռ��������ʵ���ɫ��״̬��
��7��װ��G��������
��8����Gװ�õ�����β������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��1��ʵ��ǰ��������еIJ����ǣ���������ƣ�����д������̣�____________________��
��2����Aװ���м���Na2SO3�����ͬʱ������Ӽ���ˮ��Ȼ���ٵμ�Ũ���ᡣ�Ӽ���ˮ��������_____________________________________________________________________��
��3��С�Թ�C��������_____________________________________________________��
��4�����ƿD��ʢ���Լ���__________________________________________________��
װ��D�����������ǣ�
��_________________________________________________________________��
��_________________________________________________________________��
��_________________________________________________________________��
��5��ʵ���е�Cr2O3�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȣ��Է��¶ȹ��ߣ���������ԭ����________________________________��
��6��װ��F��U�ι����ռ��������ʵ���ɫ��״̬��____________��
��7��װ��G��������________________________________________��
��8����Gװ�õ�����β������������________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
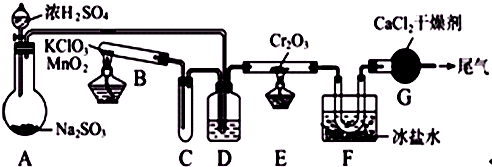
ʵ�����и���2SO2+O2![]() 2SO3����H=-196.6 kJ?mol-1�����ͼ��ʾʵ��װ�����Ʊ�SO3���塣������������⣺
2SO3����H=-196.6 kJ?mol-1�����ͼ��ʾʵ��װ�����Ʊ�SO3���塣������������⣺

��1��ʵ��ǰ��������еIJ����ǣ���������ƣ�����д������̣�____________________��
��2����Aװ���м���Na2SO3�����ͬʱ������Ӽ���ˮ��Ȼ���ٵμ�Ũ���ᡣ�Ӽ���ˮ��������______________________________________________________________________��
��3��С�Թ�C��������______________________________________________________��
��4�����ƿD��ʢ���Լ���__________________________________________________��
װ��D�����������ǣ�
��_________________________________________________________________��
��_________________________________________________________________��
��_________________________________________________________________��
��5��ʵ���е�Cr2O3�������ʱ��Ӧ���ƾ����ƿ�һ����ټ��ȣ��Է��¶ȹ��ߣ���������ԭ����________________________________��
��6��װ��F��U�ι����ռ��������ʵ���ɫ��״̬��____________��
��7��װ��G��������________________________________________��
��8����Gװ�õ�����β������������________________________________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com