
ЎҫМвДҝЎҝФЪ3ёцМе»эҫщОӘ1 LөДәгИЭГЬұХИЭЖчЦР·ўЙъ·ҙУҰЈәSO2(g)Ј«2NO(g)![]() 2NO2(g)Ј«S(s)ЎЈёДұдИЭЖчIөД·ҙУҰОВ¶ИЈ¬ЖҪәвКұc( NO2)УлОВ¶ИөД№ШПөИзПВНјЛщКҫЎЈПВБРЛө·ЁХэИ·өДКЗ
2NO2(g)Ј«S(s)ЎЈёДұдИЭЖчIөД·ҙУҰОВ¶ИЈ¬ЖҪәвКұc( NO2)УлОВ¶ИөД№ШПөИзПВНјЛщКҫЎЈПВБРЛө·ЁХэИ·өДКЗ

ИЭЖч ұаәЕ | ОВ¶И/K | ЖрКјОпЦКөДБҝ/mol | |||
SO2 | NO | NO2 | S | ||
ўс | 0.5 | 0.6 | 0 | 0 | |
ўт | T1 | 0.5 | 1 | 0.5 | 1 |
ўу | T2 | 0.5 | 0.2 | 1 | 1 |
A. ёГ·ҙУҰөДҰӨH<0
B. T1КұЈ¬ёГ·ҙУҰөДЖҪәвіЈКэОӘ![]()
C. ИЭЖчўсУлИЭЖчўтҫщФЪT1КұҙпөҪЖҪәвЈ¬ЧЬС№ЗҝЦ®ұИРЎУЪ1:2
D. ИфT2<T1Ј¬ҙпөҪЖҪәвКұЈ¬ИЭЖчўуЦРNOөДМе»э·ЦКэРЎУЪ40%
Ўҫҙр°ёЎҝAD
ЎҫҪвОцЎҝAЈ®ёщҫЭНјПуЈ¬ОВ¶ИЙэёЯЈ¬ЖҪәвКұNO2ЕЁ¶ИҪөөНЈ¬ЛөГчОВ¶ИЙэёЯҝЙК№»ҜС§ЖҪәвДжПтЈ»
BЈ®T1ОВ¶И·ҙУҰҙпөҪЖҪәвКұcЈЁNO2Ј©=0.2mol/LЈ¬ёщҫЭ·ҙУҰ·ҪіМКҪјЖЛгЈ»
CЈ®ёщҫЭpV=nRT·ЦОцЈ¬ИЭЖчИЭ»эәН·ҙУҰОВ¶ИТ»¶ЁЈ¬МеПөЧЬС№ЗҝУлМеПөЦР»мәПЖшМеөДЧЬОпЦКөДБҝіЙХэұИЈ»
DЈ®ОВ¶ИҪөөНЈ¬УРЦъУЪ»ҜС§·ҙУҰХэПтҪшРРЈ¬ёщҫЭ·ҙУҰ·ҪіМКҪәНөИР§ЖҪәвөДЦӘК¶·ЦОцЎЈ
AЈ®ёщҫЭНјПуЈ¬ОВ¶ИЙэёЯЈ¬ЖҪәвКұNO2ЕЁ¶ИҪөөНЈ¬ЛөГчОВ¶ИЙэёЯҝЙК№»ҜС§ЖҪәвДжПтЈ¬ТтҙЛХэ·ҙУҰОӘ·ЕИИ·ҙУҰЈ¬јҙЎчHЈј0Ј¬AХэИ·Ј»
BЈ®T1ОВ¶И·ҙУҰҙпөҪЖҪәвКұcЈЁNO2Ј©=0.2mol/LЈ¬ФтЖҪәвКұcЈЁSO2Ј©=0.5mol/L-0.1mol/L=0.4mol/LЈ¬cЈЁNOЈ©=0.6mol/L-0.2mol/L=0.4mol/LЈ¬ЛщТФ·ҙУҰөД»ҜС§ЖҪәвіЈКэОӘK=c2(NO2)/c2(NO)c(SO2)=
0.22/0.42ЎБ0.4=5/8Ј¬BҙнОуЈ»
CЈ®ёщҫЭАнПлЖшМеЧҙМ¬·ҪіМpV=nRT·ЦОцЈ¬ИЭЖчИЭ»эәН·ҙУҰОВ¶ИТ»¶ЁЈ¬МеПөЧЬС№ЗҝУлМеПөЦР»мәПЖшМеөДЧЬОпЦКөДБҝіЙХэұИЈ¬ИЭЖчўтПаөұУЪ°ҙ0.75molSO2Ј¬1.5molNOәН0.75molSЖрКјЈ¬УЙУЪSКЗ№ММеЈ¬І»ёДұдЕЁ¶ИЙМЈ¬ЙиИЭЖчўтЦР·ҙУҰҙпөҪЖҪәвКұПыәДБЛymolSO2Ј¬ФтЖҪәвКұБҪИЭЖчС№БҰұИОӘpI/pII=(0.4+0.4+0.2+0.1)/(2y)ЈҪ1/(2y)Јҫ1/2Ј¬CҙнОуЈ»
DЈ®T2ЈјT1Ј¬ФтОВ¶ИҪөөНУРЦъУЪ»ҜС§·ҙУҰХэПтҪшРРЈ¬ИЭЖчўуПаөұУЪТФ1molSO2Ј¬1.2molNOәН0.5molSЖрКјЈ¬SІ»¶Ф»ҜС§·ҙУҰөДЖҪәвІъЙъУ°ПмЈ¬ТІҫНПаөұУЪ¶ФИЭЖчўсјУС№Ј¬ИфЖҪәвІ»·ўЙъТЖ¶ҜЈ¬ФтЖҪәвКұNOөДМе»э·ЦКэОӘ40%Ј¬¶шИЭЖчўуөД»ҜС§·ҙУҰХэПтҪшРРіМ¶ИұИИЭЖчIёьҙуЈ¬ФтҙпөҪЖҪәвКұЈ¬ИЭЖчўуЦРNOөДМе»э·ЦКэРЎУЪ40%Ј¬DХэИ·Ј¬ҙр°ёСЎADЎЈ


 ұёХҪЦРҝјә®јЩПөБРҙр°ё
ұёХҪЦРҝјә®јЩПөБРҙр°ё
| Дкј¶ | ёЯЦРҝОіМ | Дкј¶ | іхЦРҝОіМ |
| ёЯТ» | ёЯТ»Гв·СҝОіМНЖјцЈЎ | іхТ» | іхТ»Гв·СҝОіМНЖјцЈЎ |
| ёЯ¶ю | ёЯ¶юГв·СҝОіМНЖјцЈЎ | іх¶ю | іх¶юГв·СҝОіМНЖјцЈЎ |
| ёЯИэ | ёЯИэГв·СҝОіМНЖјцЈЎ | іхИэ | іхИэГв·СҝОіМНЖјцЈЎ |
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝіЈОВПВЈ®УРЕЁ¶ИҫщОӘ0.1 molЎӨL-lөДПВБР4ЦЦИЬТәЈә
ўЩNaCNИЬТә ўЪNaOHИЬТә ўЫCH3COONaИЬТә ўЬNaHCO3ИЬТә

(1)Хв4ЦЦИЬТәpHУЙҙуөҪРЎөДЛіРтКЗ____ЈЁМоРтәЕЈ©Ј¬ЖдЦРўЪУЙЛ®өзАлөДH+ЕЁ¶ИОӘ____ЎЈ
(2)ўЩЦРёчАлЧУЕЁ¶ИУЙҙуөҪРЎөДЛіРтКЗ ___ЎЈ
(3)ўЬөДЛ®ҪвЖҪәвіЈКэKh=___mol/LЎЈ
(4)ИфПтөИМе»эөДўЫәНўЬЦРөОјУСОЛбЦБіКЦРРФЈ¬ФтПыәДСОЛбөДМе»эўЫ___ ўЬЈЁМоЎ°>ЎұЎўЎ°<"ЎўЎ°=ЎұЈ©
(5)25ЎжКұЈ¬ІвөГHCNәНNaCNөД»мәПИЬТәөДpH=11Ј¬Фт![]() ФјОӘ____ЎЈПтNaCNИЬТәЦРНЁИлЙЩБҝCO2Ј¬Фт·ўЙъ·ҙУҰөДАлЧУ·ҪіМКҪОӘЈә____ЎЈ
ФјОӘ____ЎЈПтNaCNИЬТәЦРНЁИлЙЩБҝCO2Ј¬Фт·ўЙъ·ҙУҰөДАлЧУ·ҪіМКҪОӘЈә____ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝә¬Бт»ҜәПОпФЪЙъІъЙъ»оЦРУҰУГ№г·әЈ¬ҝЖС§К№УГ¶ФИЛМеҪЎҝөј°»·ҫіұЈ»ӨТвТеЦШҙуЎЈ
ЈЁ1Ј©әмҫЖЦРМнјУТ»¶ЁБҝөДSO2 ҝЙТФ·АЦ№ҫЖТәСх»ҜЈ¬ХвУҰУГБЛSO2 өД___РФЎЈ
ЈЁ2Ј©ДіЛ®МеЦРБтФӘЛШЦчТӘТФS2O32-РОКҪҙжФЪЈ¬ФЪЛбРФМхјюПВЈ¬ёГАлЧУ»бөјЦВЛ®МеЦРУР»ЖЙ«»лЧЗІўҝЙДЬУРҙМјӨРФЖшО¶ІъЙъЈ¬ФӯТтКЗ___________________________________ЎЈЈЁУГАлЧУ·ҪіМКҪЛөГчЈ©
ЈЁ3Ј©КөСйКТІЙУГөО¶Ё·ЁІв¶ЁДіЛ®СщЦРСЗБтЛбСОә¬БҝЈә
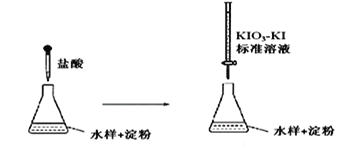
өО¶ЁКұЈ¬KIO3 әНKI ФЪСОЛбЧчУГПВОціцI2Јә5IЈӯ+ IO3Јӯ + 6H+ =3I2+3H2O
ЙъіЙөДI2 ФЩәНЛ®СщЦРөДСЗБтЛбСО·ҙУҰЈәI2 + SO32Јӯ + H2O = 2H++2IЈӯ+ SO42Јӯ
ўЩөО¶ЁөҪЦХөгКұөДПЦПуКЗ:________________________________
ўЪИфөО¶ЁЗ°КўұкЧјТәөДөО¶Ё№ЬГ»УРУГұкЧјТәИуПҙЈ¬ФтІв¶ЁҪб№ыҪ«_________ЈЁМоЎ°Ж«ҙуЎўЖ«РЎЎўІ»ұдЎұЈ©ЎЈ
ўЫөО¶ЁЦХөгКұЈ¬100mLөДЛ®Сщ№ІПыәДx mLұкЧјИЬТәЎЈИфПыәД1mLұкЧјИЬТәПаөұУЪSO32ЈӯөДЦКБҝ1gЈ¬ФтёГЛ®СщЦРSO32ЈӯөДә¬БҝОӘ__________g / L
ЈЁ4Ј©ТСЦӘ·ЗҪрКфөҘЦКБтЈЁSЈ©КЗөӯ»ЖЙ«№ММе·ЫД©Ј¬ДСИЬУЪЛ®Ј®ОӘБЛСйЦӨВИФӘЛШөД·ЗҪрКфРФұИБтФӘЛШөД·ЗҪрКфРФЗҝЈ¬Ді»ҜС§КөСйРЎЧйЙијЖБЛИзПВКөСйЈ¬Зл»ШҙрПВБРОКМвЈә
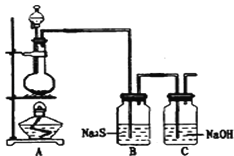
ўЩИфЧ°ЦГAөДФІөЧЙХЖҝЦРКўЧ°¶юСх»ҜГМЈ¬Фт·ЦТәВ©¶·ЦРКўЧ°өДКФјБКЗ_____________________
ўЪЧ°ЦГBЦРКөСйПЦПуОӘ___________________________Ј¬ЦӨГчВИФӘЛШөД·ЗҪрКфРФұИБтФӘЛШөД·ЗҪрКфРФЗҝЎЈ
ўЫЧ°ЦГCЦР·ҙУҰөДЧчУГКЗЈә____________________________
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПтКӘ·ЁБ¶РҝөДөзҪвТәЦРН¬КұјУИлCuәНCuSO4Ј¬ҝЙЙъіЙCuClіБөніэИҘClЎӘЈ¬ҪөөН¶ФөзҪвөДУ°ПмЈ¬·ҙУҰФӯАнИзПВЈә
Cu(s)+Cu2+(aq)![]() 2Cu+(aq) ҰӨH1ЈҪa kJЎӨmol-1
2Cu+(aq) ҰӨH1ЈҪa kJЎӨmol-1
ClЎӘ(aq)+Cu+(aq)![]() CuCl(s) ҰӨH2ЈҪb kJЎӨmol-1
CuCl(s) ҰӨH2ЈҪb kJЎӨmol-1
КөСйІвөГөзҪвТәpH¶ФИЬТәЦРІРБфc(ClЎӘ)өДУ°ПмИзНјЛщКҫЎЈПВБРЛө·ЁХэИ·өДКЗ

A. ИЬТәpHФҪҙуЈ¬Ksp(CuCl)Фцҙу
B. ПтөзҪвТәЦРјУИлПЎБтЛбЈ¬УРАыУЪCl-өДИҘіэ
C. ·ҙУҰҙпөҪЖҪәвФцҙуc(Cu2+)Ј¬c(ClЎӘ)јхРЎ
D. ![]() Cu(s)+
Cu(s)+![]() Cu2+(aq)+ClЎӘ(aq)
Cu2+(aq)+ClЎӘ(aq)![]() CuCl(s)өДҰӨHЈҪ(a+2b) kJЎӨmol-1
CuCl(s)өДҰӨHЈҪ(a+2b) kJЎӨmol-1
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝПВБР·ҙУҰКфУЪСх»Ҝ»№Фӯ·ҙУҰөДКЗЈә
A. NH3 + HCl = NH4Cl B. CuO + H2![]() Cu + H2O
Cu + H2O
C. CaCO3 ![]() CaO + CO2Ўь D. H2SO4 + 2NaOH = Na2SO4 + 2H2O
CaO + CO2Ўь D. H2SO4 + 2NaOH = Na2SO4 + 2H2O
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝДі»ҜѧСЧйЧцНкёЯОВПВМъУлЛ®ХфЖш·ҙУҰөДКөСйәуөГөҪТ»ЦЦәЪЙ«·ЫД©ЎЈЛыГЗОӘМҪҫҝёГәЪЙ«·ЫД©ЦРКЗ·сУРОҙ·ҙУҰНкөДМъ·ЫЈ¬УЦҪшРРБЛИзПВКөСйЈ¬Ч°ЦГИзНј1ЛщКҫЎЈЗл»ШҙрПВБРОКМвЈә
(1)МъУлЛ®ХфЖш·ҙУҰөД»ҜС§·ҪіМКҪКЗ_____________________Ј»
(2)°ҙНј1Б¬ҪУәГТЗЖчәуЈ¬јмІйЧ°ЦГөДЖшГЬРФөДІЩЧч·Ҫ·ЁКЗ_______________Ј»
(3)ТЗЖчbөДГыіЖКЗ_____Ј¬НщТЗЖчbЦРјУИлөДКФјБҝЙДЬКЗ______(МоТ»ЦЦ)Ј»
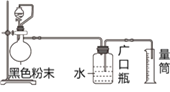
(4)КөСйЦР№ЫІмөҪЙХЖҝЦРУРЖшЕЭІъЙъЈ¬ФтәЪЙ«·ЫД©ЦР_______(МоЎ°УРЎұ»тЎ°ОЮЎұ)Мъ·ЫЈ¬ІъЙъЖшЕЭөДАлЧУ·ҪіМКҪКЗ________________________Ј»
(5)Из№ыәЪЙ«·ЫД©өДЦКБҝОӘw gЈ¬өұКұКөСйМхјюПВІъЙъөДЖшМеөДГЬ¶ИОӘҰСg/cm3 Ј¬¶аҙОКФСйЗуіцБҝНІЦРТәМеЖҪҫщМе»эОӘa mLЈ¬ФтәЪЙ«·ЫД©ЦРМъөДСх»ҜОпөДЦКБҝ·ЦКэОӘ__________________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
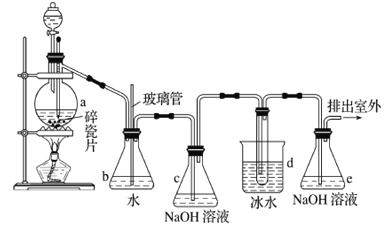
ЎҫМвДҝЎҝ1Ј¬2-¶юдеТТНйҝЙЧчҝ№ұ¬јБөДМнјУјБЎЈИзНјОӘКөСйКТЦЖұё1Ј¬2-¶юдеТТНйөДЧ°БDНјЈ¬ НјЦР·ЦТәВ©¶·әНЙХЖҝaЦР·ЦұрЧ°УРЕЁH2SO4әНОЮЛ®ТТҙјЈ¬dЧ°БDКФ№ЬЦРЧ°УРТәдеЎЈ
ТСЦӘЈәCH3CH2OH![]() CH2=CH2Ўь+H2OЈ»2CH3CH2OH
CH2=CH2Ўь+H2OЈ»2CH3CH2OH![]() CH3CH2OCH2CH3+H2O
CH3CH2OCH2CH3+H2O
Па№ШКэҫЭБРұнИзПВЈә
ТТҙј | 1Ј¬2-¶юдеТТНй | ТТГС | де | |
ЧҙМ¬ | ОЮЙ«ТәМе | ОЮЙ«ТәМе | ОЮЙ«ТәМе | әмЧШЙ«ТәМе |
ГЬ¶И/gЎӨcm-3 | 0.79 | 2.18 | 0.71 | 3.10 |
·Рөг/Ўж | 78.5 | 131.4 | 34.6 | 58.8 |
ИЫөг/Ўж | -114.3 | 9.79 | - 116.2 | -7.2 |
Л®ИЬРФ | »мИЬ | ДСИЬ | ОўИЬ | ҝЙИЬ |
ЈЁ1Ј©КөСйЦРУҰСёЛЩҪ«ОВ¶ИЙэёЯөҪ170ЎжЧуУТөДФӯТтКЗ______________________________ЎЈ
ЈЁ2Ј©°ІИ«ЖҝbФЪКөСйЦРУР¶аЦШЧчУГЎЈЖдТ»ҝЙТФјмІйКөСйҪшРРЦРdЧ°БDЦРөј№ЬКЗ·с·ўЙъ¶ВИыЈ¬ЗлРҙіц·ўЙъ¶ВИыКұЖҝbЦРөДПЦПуЈә_______________________________Ј»Из№ыКөСйКұdЧ°БDЦРөј№Ь¶ВИыЈ¬ДгИПОӘҝЙДЬөДФӯТтКЗ_______________________________________________Ј»°ІИ«Жҝb»№ҝЙТФЖрөҪөДЧчУГКЗ__________________ЎЈ
ЈЁ3Ј©ИЭЖчcЎўeЦР¶јКўУРNaOHИЬТәЈ¬cЦРNaOHИЬТәөДЧчУГКЗ________________________________ЎЈ
ЈЁ4Ј©іэИҘІъОпЦРЙЩБҝОҙ·ҙУҰөДBr2ә󣬻№ә¬УРөДЦчТӘФУЦКОӘ___________Ј¬ТӘҪшТ»ІҪМбҙҝЈ¬ПВБРІЩЧчЦРұШРиөДКЗ_____________ ЈЁМоЧЦДёЈ©ЎЈ
AЈ®ЦШҪбҫ§ BЈ®№эВЛ CЈ®ХфБу DЈ®ЭНИЎ
ЈЁ5Ј©КөСйЦРТІҝЙТФі·ИҘdЧ°БDЦРКўұщЛ®өДЙХұӯЈ¬ёДОӘҪ«АдЛ®ЦұҪУјУИлөҪdЧ°БDөДКФ№ЬЦРЈ¬ФтҙЛКұАдЛ®іэБЛДЬЖрөҪАдИҙ1Ј¬2-¶юдеТТНйөДЧчУГНвЈ¬»№ҝЙТФЖрөҪөДЧчУГКЗ____________________________ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝБтхЈВИ(SO2Cl2)іЈЧчВИ»ҜјБ»тВИ»З»ҜјБЈ¬УГУЪЦЖЧчТ©Ж·ЎўИҫБПЎўұнГж»оРФјБөИЎЈУР№ШОпЦКөДІҝ·ЦРФЦКИзПВұнЈә
ОпЦК | ИЫөг/Ўж | ·Рөг/Ўж | ЖдЛьРФЦК |
SO2Cl2 | Јӯ54.1 | 69.1 | ўЩТЧУлЛ®·ҙУҰЈ¬ІъЙъҙуБҝ°ЧОн ўЪТЧ·ЦҪвЈәSO2Cl2 |
H2SO4 | 10.4 | 338 | ОьЛ®РФЗТІ»ТЧ·ЦҪв |
КөСйКТУГёЙФп¶шҙҝҫ»өД¶юСх»ҜБтәНВИЖшәПіЙБтхЈВИЈ¬Ч°ЦГИзНјЛщКҫ(јРіЦТЗЖчТСКЎВФ)Ј¬Зл»ШҙрУР№ШОКМвЈә
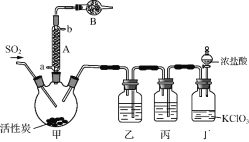
(1) ТЗЖчAАдИҙЛ®өДҪшҝЪ_______(МоЎ°aЎұ»тЎ°bЎұ)ЎЈ
(2) ТЗЖчBЦРКў·ЕөДТ©Ж·КЗ_______ЎЈ
(3) КөСйЛщРи¶юСх»ҜБтУГСЗБтЛбДЖУлБтЛбЦЖұёЈ¬ёГ·ҙУҰөДАлЧУ·ҪіМКҪОӘ_______Ј¬ТФПВУл¶юСх»ҜБтУР№ШөДЛө·ЁЦРХэИ·өДКЗ_______ЎЈ
A.ТтОӘSO2ҫЯУРЖҜ°ЧРФЈ¬ЛщТФЛьДЬК№Ж·әмИЬТәЎўдеЛ®ЎўЛбРФKMnO4ИЬТәЎўКҜИпКФТәНКЙ«
B.ДЬК№Ж·әмИЬТәНКЙ«өДОпЦКІ»Т»¶ЁКЗSO2
C.SO2ЎўЖҜ°Ч·ЫЎў»оРФМҝЎўNa2O2¶јДЬК№әмД«Л®НКЙ«Ј¬ЗТФӯАнПаН¬
D.өИОпЦКөДБҝөДSO2әНCl2»мәПәуНЁИлЧ°УРКӘИуөДУРЙ«ІјМхөДјҜЖшЖҝЦРЈ¬ЖҜ°ЧР§№ыёьәГ
E.ҝЙУГЕЁБтЛбёЙФпSO2
F.ҝЙУГіОЗеөДКҜ»ТЛ®јшұрSO2әНCO2
(4) Ч°ЦГұыЛщКўКФјБОӘ_______Ј¬ИфИұЙЩЧ°ЦГТТЈ¬ФтБтхЈВИ»бЛрК§Ј¬ёГ·ҙУҰөД»ҜС§·ҪіМОӘ______________ЎЈ
(5) ЙЩБҝБтхЈВИТІҝЙУГВИ»ЗЛб(ClSO3H)·ЦҪв»сөГЈ¬ёГ·ҙУҰөД»ҜС§·ҪіМКҪОӘЈә2ClSO3H===H2SO4Ј«SO2Cl2Ј¬ҙЛ·Ҫ·ЁөГөҪөДІъЖ·ЦР»б»мУРБтЛбЎЈ
ўЩҙУ·ЦҪвІъОпЦР·ЦАліцБтхЈВИөД·Ҫ·ЁКЗ______________ЎЈ
ўЪЗлЙијЖКөСй·Ҫ°ёјмСйІъЖ·ЦРУРБтЛб(ҝЙСЎКФјБЈәПЎСОЛбЎўПЎПхЛбЎўBaCl2ИЬТәЎўХфБуЛ®ЎўКҜИпИЬТә)____ЎЈ
Ійҝҙҙр°ёәНҪвОц>>
ҝЖДҝЈәёЯЦР»ҜС§ АҙФҙЈә МвРНЈә
ЎҫМвДҝЎҝ4ЦЦПаБЪЦчЧе¶МЦЬЖЪФӘЛШөДПа¶ФО»ЦГИзұнЈ¬ФӘЛШXөДФӯЧУәЛНвөзЧУКэКЗMөД2ұ¶Ј¬YөДСх»ҜОпҫЯУРБҪРФЎЈ»ШҙрПВБРОКМвЈә
![]()
ЈЁ1Ј©ФӘЛШXФЪЦЬЖЪұнЦРөДО»ЦГКЗөЪ________ЦЬЖЪЎўөЪ________ЧеЈ¬ЖдөҘЦКҝЙІЙУГөзҪвИЫИЪ________өД·Ҫ·ЁЦЖұёЎЈ
ЈЁ2Ј©MЎўNЎўYИэЦЦФӘЛШЧоёЯјЫСх»ҜОп¶ФУҰөДЛ®»ҜОпЦРЈ¬ЛбРФЧоЗҝөДКЗ____________Ј¬јоРФЧоЗҝөДКЗ______ЎЈ(Мо»ҜС§КҪ)
ЈЁ3Ј©ЖшМе·ЦЧУ(MN)2өДөзЧУКҪОӘ___________ЎЈ(MN)2іЖОӘДвВұЛШЈ¬РФЦКУлВұЛШАаЛЖЈ¬ЖдУлЗвСх»ҜДЖИЬТә·ҙУҰөД»ҜС§·ҪіМКҪОӘ________
Ійҝҙҙр°ёәНҪвОц>>
°Щ¶ИЦВРЕ - Б·П°ІбБРұн - КФМвБРұн
әюұұКЎ»ҘБӘНшОҘ·ЁәНІ»БјРЕПўҫЩұЁЖҪМЁ | НшЙПУРәҰРЕПўҫЩұЁЧЁЗш | өзРЕХ©ЖӯҫЩұЁЧЁЗш | ЙжАъК·РйОЮЦчТеУРәҰРЕПўҫЩұЁЧЁЗш | ЙжЖуЗЦИЁҫЩұЁЧЁЗш
ОҘ·ЁәНІ»БјРЕПўҫЩұЁөз»°Јә027-86699610 ҫЩұЁУКПдЈә58377363@163.com