
| A�� | ԭ��Һ��һ��������Ba2+��HCO3- | |
| B�� | ȡ������Һ����KSCN����Һ�Ժ�ɫ����ԭ��Һһ����Fe3+ | |
| C�� | ԭ��Һ��һ������SO42-��Cl- | |
| D�� | Ϊȷ��ԭ��Һ���Ƿ���K+����ͨ����ɫ��Ӧֱ�ӹ۲���ɫ�Ƿ�Ϊ��ɫ��ȷ�� |
���� ���ò�����պȡԭ��Һ����pH��ֽ�ϣ���ֽ�Ժ�ɫ������Һ�����ԣ���һ��������HCO3-����Һ�п��ܴ���NH4+��Fe2+��Fe3+��
����ȡ����ԭ��Һ����BaCl2��Һ�����ɲ�����ϡ����İ�ɫ�������ð�ɫ����Ϊ���ᱵ������Һ��һ������SO42-���������ӹ�����һ��������Ba2+��
��ȡ�����ϲ���Һ�����ữ����������Һ�����ɰ�ɫ���������ڢ��м����Ȼ�����Һ�����������ӣ������ж�ԭ��Һ���Ƿ���Cl-��
���ݷ�����֪����Һ��һ��������Ba2+��HCO3-��һ������SO42-����ȷ���Ƿ���Cl-��
�ݴ˽��н��
��� �⣺���ݢٿ�֪��Һ�����ԣ���һ��������HCO3-����Һ�п��ܴ���NH4+��Fe2+��Fe3+�����ݢڿ�֪��Һ��һ������SO42-���������ӹ�����һ��������Ba2+��
���ڢ��м����Ȼ�����Һ�����������ӣ�����ݢ����ж�ԭ��Һ���Ƿ���Cl-��
A�����ݷ�����֪����Һ��һ��������Ba2+��HCO3-����A��ȷ��
B��ȡ������Һ����KSCN����Һ�Ժ�ɫ��˵��������Һ��һ����Fe3+���������ڼ������ᣬ�������ӻᱻ�����������ӣ��������ж�ԭ��Һ���Ƿ���Fe3+����B����
C��ԭ��Һ��һ������SO42-����ȷ���Ƿ���Cl-����C����
D��ȷ��ԭ��Һ���Ƿ���K+����ͨ����ɫ��Ӧ����ɫ�ܲ����۲���ɫ�Ƿ�Ϊ��ɫ��ȷ������D����
��ѡA��
���� ���⿼���˳������ӵ����ʼ����鷽������Ŀ�Ѷ��еȣ���ȷ�������ӵ�����Ϊ���ؼ���ȷ���Ƿ���������Ϊ�״��㣬ע����ȷ�������Ӵ���ʱ�������ų��������ӣ�ȷ�����鷽���������ԣ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | CuCl2��CuCl2��Һ�� | B�� | AgNO3��Ag2O�� | C�� | NaCl��HCl��Һ�� | D�� | CuSO4��Cu��OH��2�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ������ | B�� | ������ | C�� | ������Һ | D�� | ����������Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
| �ɷ� | Na+ | K+ | Ca2+ | Mg2+ | Cl- | SO42- | HCO3- |
| ����/mg•L-1 | 9360 | 83 | 200 | 1100 | 16000 | 1200 | 118 |
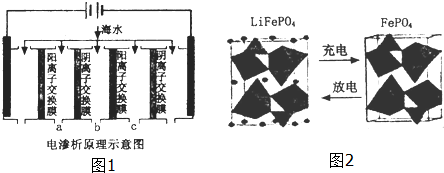
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
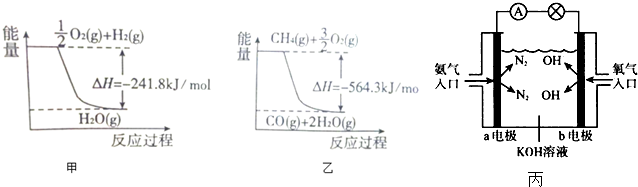
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �Ȼ��Ƶľ���ģ�ͣ�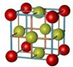 | |
| B�� | ��ԭ�������ĵ�����ͼ�� | |
| C�� | ������Ľṹʽ��H-O-Cl | |
| D�� | CH3CHOHCH��CH3��2���ƣ�2-3-�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
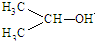 �ɼ�дΪ
�ɼ�дΪ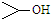 �����������������֯�Ƽ��Ŀ�������ҩ���ṹ��ʽ��ͼ��
�����������������֯�Ƽ��Ŀ�������ҩ���ṹ��ʽ��ͼ��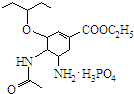 �����й�����˵��������ǣ�������
�����й�����˵��������ǣ�������| A�� | �����һ�������� | |
| B�� | ��Ʒ����к����ļ� | |
| C�� | ��Ƶķ���ʽΪC16H31N2O8P | |
| D�� | 1mol��ƿ���2mol H2�����ӳɷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 28g N2��N2O��N2O4�Ļ�����庬�е�ԭ����Ϊ2NA | |
| B�� | ������Ϊ7.8 g Na2S��Na2O2�Ĺ����к��е�����������Ϊ0.1NA | |
| C�� | 0.5 mol•L-1��������Һ�У�������ӵ���ĿС��0.5NA | |
| D�� | 78g�������к���̼̼˫����Ϊ3 NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������C��D�ֱ�Ϊ�κ�ˮ����÷�Ӧһ�����кͷ�Ӧ | |
| B�� | ��A��C�ǵ��ʣ�B��D�ǻ������÷�Ӧһ�����û���Ӧ | |
| C�� | ��A�ǿ����ԼB�ǿ������Σ���C��D�����������ֳ��� | |
| D�� | ��A�ǿ����ԼB�ǿ������Σ���C��Dһ������һ�ּ����һ���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com