
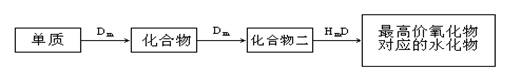
10�֣�A��B��C��D��E��λ�ڶ����ڵ�����Ԫ�أ�����A��BΪ����Ԫ�ء���֪�������ȶ��ԣ�HmD��HmC����Cm����E��m��1��-������ͬ�ĵ��Ӳ�ṹ����B��E��ͬһ���ڣ��ڸ������У�E��ԭ�Ӱ뾶��С��B�����Ӱ뾶��С����A��B���γɵĵ������Ӿ�����ͬ�ĵ��Ӳ�ṹ��B������������Ӧ��ˮ������A��E������������Ӧ��ˮ���ﶼ�ܷ�Ӧ������������Ϣ�ش��������⣺��1��HmD�ĵ���ʽ��___________________��
��2��Cm����E��m��1��-�Ļ�ԭ��ǿ��Ϊ___________��___________�������ӷ��ţ�����֤����
��ԭ��ǿ�������ӷ���ʽΪ ��
��3��д��B������������Ӧ��ˮ�����A������������Ӧ��ˮ���ﷴӦ�����ӷ���ʽ_________________________________________________________��
��4����B��C��E�����У���������ת����ϵ����___________������Ԫ�ط��ţ�

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)��A��B��C��D��E��F����Ԫ�أ���֪������λ��������ͬ�����ڣ��˵������������A��E��B��F�ֱ�ͬ���壻A��E�ֱ�����D��ԭ�Ӹ�����1�s1��2�sl�γɻ����B��C�ֱ�����D��ԭ�Ӹ�����1�s1��1�s2�γɻ����
��д��ֻ����A��B��D��E����Ԫ�ص����ֳ�����ˮ�εĻ�ѧʽ_____________��
����ͼ��A��D�γɵ�һ�ֻ�����ľ���ṹʾ��ͼ�����е����߱�ʾ__________��E��D��ԭ�Ӹ�����1�s1�γɵĻ�����ĵ���ʽΪ_____________________��
������ͨ���Ѳ�l molij��ѧ�������յ��������ɸ�
��ѧ���ļ��ܡ����ܵĴ�С���Ժ�����ѧ����ǿ����Ҳ����
 ���ڹ��㻯ѧ��Ӧ�ķ�Ӧ��(��H)����ѧ��Ӧ�ġ�H���ڷ�Ӧ
���ڹ��㻯ѧ��Ӧ�ķ�Ӧ��(��H)����ѧ��Ӧ�ġ�H���ڷ�Ӧ
�ж��Ѿɻ�ѧ���ļ���֮���뷴Ӧ���γ��»�ѧ���ļ���֮��
�IJ�±��г�����������Ԫ���γɵĻ�ѧ���ļ��ܣ�
| ��ѧ�� | F��D | F��F | B��B | F��B | C�TD | D�TD |
| ����/kJ��mol��1 | 460 | 176 | 347.7 | 347 | 745 | 497.3 |
���������������γɵľ��壬�۵��ɸߵ��͵�˳��(��a��b��c��ʾ)��_________��
a��F��B�γɵĻ����� b��B��D�γɵ��ȶ������� c��F�ĵ���
���Թ���F���ʾ����ȼ���ȣ�____________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��10��)��A��B��C��D��E���ֳ���������������±��е������γɵģ�
| ������ | K+ Na+ Cu2+ Al3+ |
| ������ | SO42�� HCO3�� NO3�� OH�� |
Ϊ�˼�������������ֱ��������ʵ�飬�����ǣ�
�ٽ���������ˮ��DΪ��ɫ��Һ��������Ϊ��ɫ��Һ��
�ڽ�E��Һ���뵽C��Һ�г��ְ�ɫ�����������μӣ������ܽ⣻
�۽�����ɫ��Ӧ��ֻ��B��CΪ��ɫ(����ɫ�겣��)��
���ڸ���Һ�м������ᱵ��Һ���ټӹ���ϡ���ᣬA�зų���ɫ���壬C��D�в���
��ɫ������
�ݽ�B��D����Һ��ϣ�δ���������������ɣ�
��������ʵ����գ�
(1)д��B��D�Ļ�ѧʽ��B ��D ��
(2)����1molA����Һ�뺬1molE����Һ��Ӧ�����ɣ����õ�һ�ֻ�����û�����Ļ�ѧʽΪ ��
(3)��A��Һ�м�����������ʯ��ˮ�������ӷ���ʽΪ ��
(4)C��������ˮ���������ӷ���ʽ��ʾ�侻ˮԭ�� ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����10�֣�A��B��C��D��E��F��G����ѧ�ﳣ������һЩ���ʣ�����֮��������ת����ϵ����Щ��Ҫ����Ϣ���ڿ�ͼ��ע����
�ش��������⣺
��1��D�Ŀռ乹��Ϊ ��C�ĵ���ʽΪ ��F��Һ������ ��
��2����ʵ��������ȡ����A�����ӷ���ʽ�� ��
��3��д��A��D��C�Ļ�ѧ����ʽ ������������
�������뻹ԭ�����ʵ���֮��Ϊ ��
��4��д����F��B(����)��E�����ӷ���ʽ ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2014�����ɹź��ױ����и�һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
��10��)����A��B��C��D���ֿ�������,���ǵ������ӷֱ������Ba2+��Ag+��Na+��Cu2+�е�ijһ�֣��������������NO3-�� SO42-�� Cl-��CO32-�����е�ijһ�֡�
(1)�����������ܽ�����֧�Թ��У�ֻ��C����Һ����ɫ��
(2)����1������֧�Թ��зֱ�������ᣬB����Һ���г������ɣ�D����Һ������ɫ��ζ����ų���
��������ʵ����ʵ�ƶ����ǵĻ�ѧʽΪ��A B C D
д��B�������ᷴӦ�����ӷ���ʽ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com