
(12��) A��B��W��D��EΪ������Ԫ�أ���ԭ��������������������֮��Ϊ39��B��Wͬ���ڣ�A��Dͬ���壬A��W���γ�����Һ̬������A2W��A2W2��EԪ�ص���������������������ȡ�
��1��EԪ�������ڱ��е�λ��Ϊ ��
��2����A��B��W����Ԫ����ɵ�18�������ĵ���ʽΪ ��
��3�����ⶨA2W2Ϊ��Ԫ���ᣬ�����Ա�̼��Ļ�Ҫ������д�����һ������ĵ��뷽��ʽ ���������ᴦ��BaO2���Ʊ�A2W2��д���÷�Ӧ�Ļ�ѧ����ʽ ��
��4����ӡˢ��·���Ϻ���ͭ�������Ļ��շ����ǽ�������ʹͭת��Ϊ����ͭ�����������ܽ⡣�ָ���A2W2��ϡ������ݷ�ӡˢ��·��ȴﵽ����Ŀ�ģ��ֱ����˻�������д����Ӧ�����ӷ���ʽ ��
��5��Ԫ��D�ĵ�����һ�������£�����A���ʻ�������һ���Ȼ���DA���۵�Ϊ800��DA����ˮ��Ӧ������������1molDA��1molE���ʻ�ϼ���������ˮ����ַ�Ӧ�����ȫ������� ����״���£���
��6��D��ij������ʵ���ɫ�������Ȼ�������Һ��Ӧ��������ɫ�������Ȼ�������Ӧ�����ʵ���֮��Ϊ1��2�������������ɣ���÷�Ӧ�����ӷ���ʽΪ ��
��1���������ڣ�IIIA�壻��2��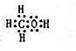
��3��H2O2  H++HO2-��BaO2+H2SO4=BaSO4��+H2O2��
H++HO2-��BaO2+H2SO4=BaSO4��+H2O2��
��4��Cu+ H++H2O2=Cu2++2H2O ��5��56L
��6��3Na2O2+6Fe2++6H2O=4Fe(OH)3��+6Na++2Fe3+
��������
���������Ԫ���ƶϣ�A��B��W��D��EΪ������Ԫ�أ���ԭ����������������A��W���γ�����Һ̬������A2W��A2W2֪AΪH��WΪO��������֪DΪNa��EԪ�ص���������������������ȣ���֪ΪAl��������Ԫ��������֮��Ϊ39��֪BΪCԪ�أ�
��2����A��B��W����Ԫ����ɵ�18����������H��C��O�����ߵ������ֱ�Ϊ1��6��8��������Ϊ15��ֻ���ټ�������ԭ�ӣ���18������Ӧ��ΪCH4O��Ҳ���Ǽ״�������ʽΪ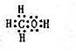 ��
��
��3��H2O2��һ�����뷽��ʽΪ��H2O2  H++HO2-������ǿ�������ᣬ��֪��BaO2+H2SO4=BaSO4��+H2O2
H++HO2-������ǿ�������ᣬ��֪��BaO2+H2SO4=BaSO4��+H2O2
��4��Cu�����Ի�������O2��Ӧ�����ӷ���ʽΪ��Cu+ H++H2O2=Cu2++2H2O��
��5���ɣ� 2Na +H2 =2NaH�� NaH + H2O = NaOH + H2���� 2Al+2NaOH+2H2O =2NaAlO2+3H2��
֪��2Al+2NaH+4H2O =2NaAlO2+5H2����������2.5molH2��Ҳ����56L��
��6��2Na2O2+2H2O=4Na++4OH�� + O2�� 4Fe2+ +O2 +2H2O=4Fe3++4OH��
��ʽ��ӣ�2Na2O2+4Fe2+ +4H2O=4Na++8OH�� + 4Fe3+
��ʽ�ұ�OH��Ӧ��ȫ������Fe(OH)3����Fe3++3OH�� = Fe(OH)3��
2Na2O2+4Fe2+ +4H2O=4Na++8/3Fe(OH)3�� + 4/3Fe3+
�����ٳ�3/2����3Na2O2+6Fe2++6H2O=4Fe(OH)3��+6Na++2Fe3+
���㣺Ԫ���������ƶ�
������������Ҫ��Ԫ�ء�λ�������ԡ����߹�ϵ���ۺϿ��飬�Ƚ�ȫ�濼��ѧ���й�Ԫ���ƶ�֪ʶ���������֪ʶ�������������ԡ����ڱ���Ԫ�ص��ƶϡ�Ϊ���壬����ѧ����Ԫ�����ڱ�����Ϥ�̶ȼ���Ա��и�Ԫ�����ʺ���Ӧԭ�ӽṹ�������Եݱ���ɵ���ʶ�����ճ̶ȡ�������ѧ�������ʽṹ�����ʹ�ϵ�Լ�����Ԫ�������ɽ�����廯ѧ�����������


 53���ò�ϵ�д�
53���ò�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)��A��B���ֳ�����������ɻ�������ɫ��Ӧ��Ϊ��ɫ�����ת����ϵ��ͼ(�������ʾ���ȥ)��
�������Ϲ�ϵ���ش��������⣺
(1)д��A��B��C��D�Ļ�ѧʽ��A_______�� B__________��C________��D________��
(2)д��������м�ˮ��Ӧ�Ļ�ѧ����ʽ��
_________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(12��)��A��B��C��D���ֶ�����Ԫ��, ���ǵ�ԭ��������A��D��������, ��֪A��Bԭ������ͬ�ĵ��Ӳ���, ��A��L���������K�������������, Cȼ��ʱ���ֻ�ɫ����, C�ĵ����ڸ�������B�ĵ��ʳ�ַ�Ӧ, ���Եõ���D������ɫ��ͬ�ĵ���ɫ��̬������, �Ը������������ش�
��1��Ԫ������: A ________ B __________ C__________ D ___________
��2��д��AԪ�������ڱ��е�λ�ã���_________���ڣ���_______�塣
��3��C �����ڸ�������B���ʳ�ַ�Ӧ�Ļ�ѧ����ʽΪ ____________��
��4���õ���ʽ��ʾ������ C2D ���γɹ��� _______________________________��
��5��һ������£���Ԫ����DԪ���γɵĻ���������____������Ի�Ǽ��ԣ���ϵ����������������ۡ������ӡ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012�����ʡ��ˮ�ж��и���ģ�⣨5�£����Ի�ѧ�Ծ����������� ���ͣ������
����2����ÿ��3�֣�����ÿ��2�֣���12�֣�A��B��C��D��Ϊ��ѧ��ѧ���������ʣ�����֮��ķ�Ӧ��ϵ����ͼ��
�ش��������⣺
��1����A�ǿ����Ե�ǿ�B����ʽ�Σ�D������ϡ���ᣬ��B�Ļ�ѧʽΪ________________���йص����ӷ���ʽΪ___________________________________ ��
��2����B�����Σ�D�ȿ����������ֿ�����NaOH��Һ��
����CΪ��ʹ����ʯ��ˮ����ǵ���ɫ��ζ���壬�÷�Ӧ�����ӷ���ʽΪ
____________________________________________________ ��
����A��һ�ֵ���ɫ���壬��A��B�����ʵ���֮��3��1���ʱ����֪B�Ļ�ѧʽΪ
_________ _��
��3����BΪCa(HCO3)2��Һ��A��B����Һ��Ӧʱֻ��������C��CaCO3��ˮ����A�Ļ�ѧʽΪ__________________ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013�찲�����������ѧ������ѧ���ص����¿���ѧ�Ծ��������棩 ���ͣ������
(12��)��A��B��C��D���ֶ����ڵķǽ���Ԫ��(�䵥��Ҳ�ɷֱ���A��B��C��D��ʾ)������Ԫ�ص�ԭ��������B��D��C��A˳������D��CԪ�������ڱ���λ�����ڡ���һ�������£�B���Էֱ��A��C��D�������ɼס��ҡ��������C��D���Ͽɵö�����֪�ҡ������������и�����10�����ӣ����Ҽס��ҡ����������������µı仯��ϵ��

����д���пո�
(1)��Ũ��Һ��һ�ֺ�ɫ��ĩ���ȿɵ�A�����Ϊ_______����Ϊ___________��
(2)��Ľṹʽ��______________��DB4+�ĵ���ʽ��_______________��
(3)��+����D��һ�������·�Ӧ����һ��������Ⱦ�ĺð취��д���÷�Ӧ�Ļ�ѧ����ʽ��
����������ת�������_____________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ� ���ͣ������
(12��)��A��B��C��D����Ԫ�أ�AԪ���γɵ�-2�������ӱȺ�ԭ�ӵĺ����������8����B Ԫ�ص�һ��������Ϊ����ɫ���壬�ù�����������������A�ĵ��ʣ�CΪԭ�Ӻ�����12�����ӵĶ��۽�������2 .4��C��������ˮ��Ӧʱ���ڱ�״���·ų�����2.24L��D��M����7�����ӡ����٢ڢ�ÿ��1�֣�
��д��A~DԪ�ط��ţ�A____,B_____,C_____,D_____
��д��A��ԭ�ӽṹʾ��ͼ�� B�����ӽṹʾ��ͼ:��
D�����ڱ���λ�ã� C��D���γɵĻ�����ĵ���ʽ
���ֱ�д��B ��D������������ˮ����Ļ�ѧʽ______ ��______
���Ƚ�D����̬�⻯����H2S��HF���ȶ��ԣ�(2��)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com