


| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��SiCl4��H2 ="=" SiHCl3��HCl��H=" +31" kJ/mol |
| B��SiCl4��H2 ="=" SiHCl3��2HCl ��H=" -31" kJ/mol |
| C��SiCl4(g)��H2(g) ="=" SiHCl3(g)��HCl(g) ��H="+31" kJ/mol |
| D��SiCl4(g)��H2(g) ="=" SiHCl3(g)��2HCl(g) ��H=" -31" kJ/mol |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��


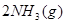 ��H=
��H=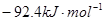 �����¡��������ݻ���ͬ�������ܱ�����A��B��A��ͨ��1molN2��3 molH 2��B ��ͨ��0.5 molN2��1.5 mol H 2����Ӧһ��ʱ���A��B�о��ﵽƽ��״̬�������ж���ȷ���ǣ� ��
�����¡��������ݻ���ͬ�������ܱ�����A��B��A��ͨ��1molN2��3 molH 2��B ��ͨ��0.5 molN2��1.5 mol H 2����Ӧһ��ʱ���A��B�о��ﵽƽ��״̬�������ж���ȷ���ǣ� ��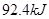

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
 2CO(g) ��H��0 ����Ӧ����Ϊ u1��N2��3H2
2CO(g) ��H��0 ����Ӧ����Ϊ u1��N2��3H2 2NH3 ��H��0��Ӧ����Ϊ u2������������Ӧ�����¶�����ʱ��u1��u2�ı仯���Ϊ �� ��
2NH3 ��H��0��Ӧ����Ϊ u2������������Ӧ�����¶�����ʱ��u1��u2�ı仯���Ϊ �� �� | A��ͬʱ���� | B��ͬʱ��С | C������С | D����С������ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��Q2>Q3>Q1 | B��Q2>Q1>Q3 | C��Q1��Q2��Q3 | D��Q2��Q3>Q1 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������

 OH)3��+�� ��H2Te��
OH)3��+�� ��H2Te���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���������غ㶨�ɣ���Ӧ���������һ������������������� |
| B���Ʋ������������ȶ�п�������ʴ |
| C���ں����������п�鱣����Dz��ܸ�ʴ�Dz������������������������� |
| D�����������������������ֱ���ȫȼ�գ����߷ų��������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ��Ҫ����д�Ȼ�ѧ����ʽ��
��Ҫ����д�Ȼ�ѧ����ʽ�� g��ɫ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ
g��ɫ������д����ʾ����ȼ���ȵ��Ȼ�ѧ����ʽ O2(g)��2CO2(g)��2H2O(l) ��H1����87
O2(g)��2CO2(g)��2H2O(l) ��H1����87 0.3 kJ/mol
0.3 kJ/mol�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| �¶�/�� | 200 | 300 | 400 | 500 | 600 |
| ������/% | 89.9 | 71.0 | 47.0 | 26.4 | 13. 8 8 |
 2NH3��2 min�ﵽƽ��״̬ʱ��H2ת������50%�����¶��µ�ƽ�ⳣ��K��______________(�÷�����ʾ)����ʹK�����Բ�ȡ�Ĵ�ʩ��___________________��
2NH3��2 min�ﵽƽ��״̬ʱ��H2ת������50%�����¶��µ�ƽ�ⳣ��K��______________(�÷�����ʾ)����ʹK�����Բ�ȡ�Ĵ�ʩ��___________________��
 2NH3(l) ��H=___________��
2NH3(l) ��H=___________���鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com