



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| A���зḻ��������Դ�ij��� |
| B���������������� |
| C��������������Ĺ�ҵ���� |
| D���˿ڳ��ܵ��Ļ�����ҵ���ij��� |
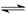 2SO3��g������H <0���ֽ�0.050 mol SO2��0.030 mol O2�����ݻ�Ϊ1 L���ܱ������У���Ӧ��һ�������´ﵽƽ�⣬��÷�Ӧ������ѹǿ��С��ԭ��ѹǿ��75%�����������SO2��ת����Ϊ ���������µ�ƽ�ⳣ��Ϊ ��
2SO3��g������H <0���ֽ�0.050 mol SO2��0.030 mol O2�����ݻ�Ϊ1 L���ܱ������У���Ӧ��һ�������´ﵽƽ�⣬��÷�Ӧ������ѹǿ��С��ԭ��ѹǿ��75%�����������SO2��ת����Ϊ ���������µ�ƽ�ⳣ��Ϊ ��| ����¯�¶�/�� | 600 | 620 | 640 |
| ¯����CuSO4��������/% | 9.3 | 9.2 | 9.0 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A���л��ﲻ���硡�� | B�����ʯ����Ȼ����Ӳ������ |
| C��SO2������ʳƷ������ | D��NO������ijЩ���������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ͭƬ�ܽ⣬��������ȴ���ˮϡ����Һ����ɫ |
| B�����ɵ��������Ϊ1.12L |
| C���μӷ�Ӧ�������뱻��ԭ���������ʵ���֮��Ϊ2:1 |
| D����ʵ���п���Na2CO3��Һ����β�� |
�鿴�𰸺ͽ���>>
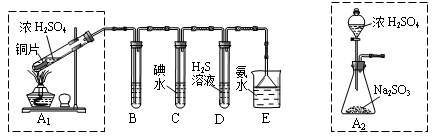
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��CO2 | B��NH3�� �� | C��NO2���� | D��H2S |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
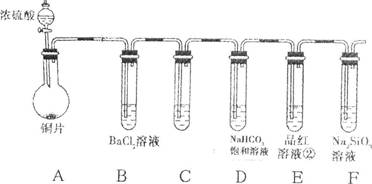
�鿴�𰸺ͽ���>>
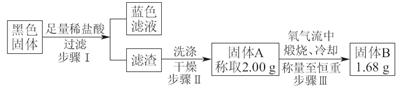
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
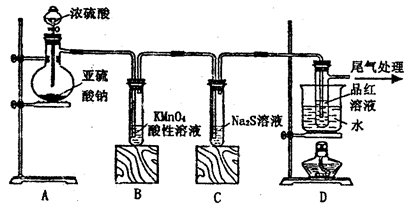
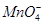 ����H2O����
����H2O����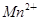 ����
����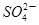 ����H����
����H�����鿴�𰸺ͽ���>>
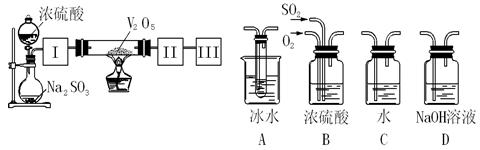
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
| ���ϣ��� H2S������ˮ��Լ1��2������ˮ��ҺΪ��Ԫ���ᡣ �� H2S��������������ӷ�Ӧ���ɳ����� �� H2S�ڿ�����ȼ�գ�����ʵ���ɫ�� |
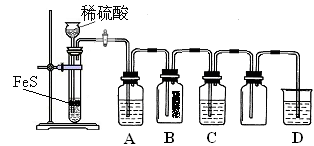
| | ʵ����� | ʵ������ |
| ʵ��1 | ����Ũ�ȵ�Na2S��Na2SO3��Һ�������2��1��� | ���������� |
| ʵ��2 | ��H2Sͨ��Na2SO3��Һ�� | δ�����Գ������ټ�������ϡ���ᣬ������������dz��ɫ���� |
| ʵ��3 | ��SO2ͨ��Na2S��Һ�� | ��dz��ɫ�������� |
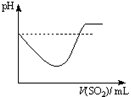 | 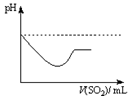 | 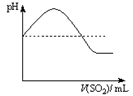 | 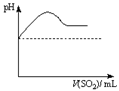 |
| A | B | C | D |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com