
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D
2-������E
2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F
4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d
84s
2��
��ش��������⣺
��1��B��C��D����Ԫ�صĵ縺���ɴ�С��˳����
O��N��C
O��N��C
����Ԫ�ط��ţ���
��2����AԪ������������Ԫ���γɵķ����У�������ԭ��Ϊsp
3�ӻ���Ϊ
CH4��H2O
CH4��H2O
����д��2�ּ��ɣ�
��3����Cͬ����λ�ڵ�������Ԫ�����γ���̬�⻯��ĵ���ʽΪ
��������
����
����
������ԡ��Ǽ��ԡ������ӣ�
��4��F�Ļ�̬ԭ�Ӻ�������Ų�ʽ��
1s22s22p63s23p63d24s2
1s22s22p63s23p63d24s2
����F�ĵ��ʾ����У�Fԭ�ӵĶѻ���ʽ��
�������ܶѻ�
�������ܶѻ�
��Fԭ�ӵ���λ����
12
12
��
��5��Eԭ�ӵĵ�һ�����ܱ�ͬ���ں�������Ԫ�صĵ�һ������
��
��
�����С������E��D�γɻ�������۵�ߣ���ԭ����
���Ӱ뾶С������������ɶ࣬�����ܴ�
���Ӱ뾶С������������ɶ࣬�����ܴ�
��
��6������������������Դ��XԪ�����磨La��Ԫ�صĺϽ����������ϣ��úϽ�ľ�����ͼ��ʾ������������һ��Xԭ�ӣ�����Xԭ�Ӷ��ھ������ϣ���þ���Ļ�ѧʽΪ
Ni5La
Ni5La
����֪�þ�����ܶ�Ϊd g?cm
-3����Ħ������ΪM g?mol
-1����þ����ı߳���
�������г���ʽ��



 ��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��
��֪��A��B��C��D��E��F��XΪ���ڱ���ǰ�����ڵ�����Ԫ�أ����ǵ�ԭ��������������A�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�B�Ļ�̬ԭ����3����ͬ���ܼ������ܼ��е�������ȣ�D�Ļ�̬ԭ��2p�ܼ��ϵ�δ�ɶԵ�������Bԭ�ӵ���ͬ��D2-������E2+���Ӿ�����ͬ���ȶ����Ӳ�ṹ��F�С����������֮�ƣ�F4+���Ӻ��ԭ�ӵĺ�������Ų���ͬ��X�Ļ�̬ԭ�ӵļ۵����Ų�ʽΪ3d84s2��

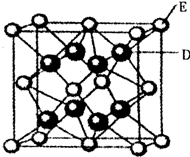 ��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�
��֪��A��B��C��D��E��F�����ڱ���ǰ36��Ԫ�أ�A��ԭ�Ӱ뾶��С��Ԫ�أ�BԪ�ػ�̬ԭ�ӵ�2P�����ֻ���������ӣ�CԪ�صĻ�̬ԭ��L��ֻ��2�ԳɶԵ��ӣ�D��Ԫ�����ڱ��е縺������Ԫ�أ�E2+�ĺ�������Ų���Arԭ����ͬ��F�ĺ˵������D��E�ĺ˵����֮�ͣ�
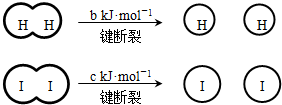 ��a��b��c�������㣩
��a��b��c�������㣩