
����Ŀ����0.8 mol I2(g)��1.2 mol H2(g)����ij1L�ܱ������У���һ���¶��·�����Ӧ��I2(g)��H2(g) ![]() 2HI(g)���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
2HI(g)���ﵽƽ�⡣HI�����������ʱ��ı仯�������ʾ��
HI������� | 1min | 2min | 3min | 4min | 5min | 6min | 7min[ |
����I | 26% | 42% | 52% | 57% | 60% | 60% | 60% |
����II | 20% | 33% | 43% | 52% | 57% | 65% | 65% |
��1��������I����ƽ��ʱ������÷�Ӧ��ƽ�ⳣ��K��Ҫ���г�������̡�
��2��������I�ӿ�ʼ��Ӧ������ƽ��ʱ��H2�ķ�Ӧ����Ϊ____________��
��3��Ϊ�ﵽ����II�����ݣ����ڷ�Ӧ��ϵ���ܸı�IJ�����_______________��
��4���÷�Ӧ����H__________0����">"��"<"��"="��
��5��������I�´ﵽƽ�����7minʱ���������ѹ��Ϊԭ����һ�롣����ͼ�л���c(HI)��ʱ��仯�����ߡ�
���𰸡���1����I2����Ũ��Ϊx
I2(g) + H2(g) ![]() 2HI(g)
2HI(g)
��ʼŨ�ȣ�mol/L����0.8 1.2 0
ת��Ũ�ȣ�mol/L����x x 2x
ƽ��Ũ�ȣ�mol/L����0.8-x 1.2-x 2x
HI���������Ϊ60%����2x/2=60%��x=0.6 mol/L
K=c2 (HI) /[c(H2)��c(I2)]=1.22/(0.2��0.6)=12��
��2��0.12 mol/(L��min)��3�������¶�����4��<��
��5��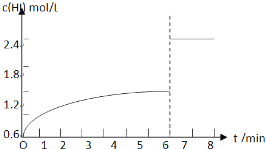
��������
�����������1���ɱ������ݿ�֪������I��5minʱ����ƽ��״̬����I2����Ũ��Ϊxmol/L����
I2��g��+H2��g��![]() 2HI��g��
2HI��g��
��ʼŨ����mol/L����0.8 1.2 0
ת��Ũ����mol/L����x x 2x
ƽ��Ũ����mol/L����0.8-x 1.2-x 2x
HI���������Ϊ60%������![]() =60%����x=0.6��ƽ�ⳣ��K= c2 (HI) /[c(H2)��c(I2)]=1.22/(0.2��0.6)=12���ʴ�Ϊ��12��
=60%����x=0.6��ƽ�ⳣ��K= c2 (HI) /[c(H2)��c(I2)]=1.22/(0.2��0.6)=12���ʴ�Ϊ��12��
��2��������I�ӿ�ʼ��Ӧ������ƽ��ʱ��H2�ķ�Ӧ����Ϊ![]() =0.12 mol/��Lmin�����ʴ�Ϊ��0.12 mol/��Lmin����
=0.12 mol/��Lmin�����ʴ�Ϊ��0.12 mol/��Lmin����
��3����ͬʱ����HI�����������С��˵����Ӧ���ʼ�����ƽ��ʱHI���������������Iʱ���ʸı�����ƽ�������ƶ�������ѹǿ��������Ӱ��ƽ���ƶ��������ǽ����¶ȣ��ʴ�Ϊ�������¶ȣ�
��4�������¶�ƽ��ʱ�����ƶ���˵������ӦΪ���ȷ�Ӧ������H��0���ʴ�Ϊ������
��5��������I�´ﵽƽ���HI��Ũ��Ϊ1.2mol/L����7minʱ���������ѹ��Ϊԭ����һ�룬ѹǿ����ƽ�ⲻ�ƶ���HI��Ũ�ȱ�Ϊԭƽ���2������HIŨ�ȱ�Ϊ2.4mol/L��c��HI����ʱ��仯������Ϊ��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��A��B��C��D��E��ԭ������������������ֶ���������Ԫ�أ�����A��ԭ��������B��Dԭ������֮�͵�1/4��CԪ�ص�����������ˮ������һ����ǿ��ͱ���DԪ�ص����ֳ������������ҺͶ���BԪ�ص����ֳ���ͬ����������0.005mol/L����Һ��pH=2������֮���ת����ϵ��ͼ��ʾ(���ַ�Ӧ��ʡ��)������������ȷ������ ��
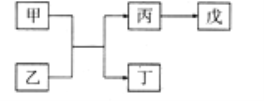
A. C��D��Ԫ���γɻ����������ۻ�����
B. C��D�ļ����Ӿ��ܴٽ�ˮ�ĵ���
C. A��D�ֱ���BԪ���γɵĻ����ﶼ�Ǵ�����Ⱦ��
D. E��������ˮ��������Դ���D��������ˮ���������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ͼ��ʾ������ƿ�зֱ�װ�뺬��̪��0.01mol/LCH3COONa��Һ�����ֱ������ʢ��ˮ���ձ��У�Ȼ�����ձ����м�����ʯ�ң����ձ����м���NH4NO3���壬�ձ����в����κ����ʣ�������������ȷ����
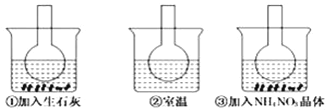
A. ��˵��ˮ�ⷴӦΪ���ȷ�Ӧ B. ��˵��ˮ�ⷴӦΪ���ȷ�Ӧ
C. ������Һ��ɫ��dz D. ������Һ��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������������ȷ����
A. ������Ư�۳���������ˮ�ľ���������������ԭ����ͬ
B. �����£�ͬŨ�ȵ�Na2S��NaHS��Һ��ȣ�Na2S��Һ��pHС
C. �����ʵ���Ũ�ȵ�NH4Cl��Һ��NH4HSO4��Һ�����ߵ�c(NH4��)��
D. FeCl3��KSCN��Ӧ�ﵽƽ��ʱ������KCl��Һ������Һ��ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����ͼ�жϣ�����������ȷ����
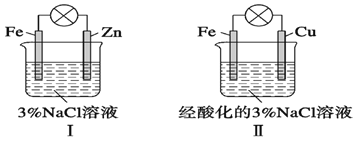
A. ��������������������
B. �������и�����Ӧ����Fe��2e��=Fe2��
C. ��������������Ӧ����O2��2H2O��4e��=4OH��
D. �������зֱ��������K3[Fe(CN)6]��Һ��������ɫ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����������ʵ�飺�ٳ�ȥ����ֲ�����е�ˮ���ڶԵ�ˮ�еĵ����Ũ�����۶�30%�ľƾ���Һ�еľƾ������ᴿ������ʵ����õ���ȷ���������ǣ� ��
A.��Һ����ȡ������B.��ȡ������Һ
C.��Һ��������ȡD.������ȡ����Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����1�������5.6L��CO2����Ϊ _____g,���к���______�����ӣ�����_____��ԭ�ӣ�
��2��������Ϊm g�� HCl��NH3��CO2��O2�������壬����������Ŀ���ٵ���_________�����������_____���ܶ���С����_______������ͬ�¶Ⱥ�ѹǿ�����£���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ˮռ�����ܴ�ˮ����97.2%�����Ѻ�ˮ�����ͻ�����������������ȿ��Խ����ˮ��Դȱ�������⣬�ֿ��Գ�����ú�����Դ��
��1��Ŀǰ������ʵ�õ�����ˮ��������Ҫ����֮һ�����������ǽ���ˮ�������������������ȴ���øߴ��ȵ�ˮ���ɴ˿��ж�������__________���������仯����ѧ�仯����
��2��ʵ������MnO2��Ũ����Ϊԭ���Ʊ��������÷�Ӧ�Ļ�ѧ����ʽΪ________________________________��������������_______��ԭ����________��β�����������ӷ���ʽΪ______________________________��
��3����ҵ���Ʊ�Ư�۵Ļ�ѧ����ʽ___________________________________��
��4����ʵ�����Ƶ�����������Ƶ���ˮ���ж����������÷���ʽ��ѧʽ�ش��������⣺
����ˮ�μ���ɫʯ����Һ�У��ȱ�����ɫ______________________________����ѧ����ʽ�����У�������ɫ����Ϊ��_________����ѧʽ�����ɾ���Ư���ԣ�
����ˮ������������Һ�У��а�ɫ��������__________________________�������ӷ���ʽ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com