
| ���� | ������ƽ�������룩 | С�ձ� | ����ǯ | ������ | ҩ�� | ��Ͳ |
| ���� | 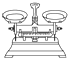 | 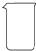 |  |  |  |  |
| ��� | a | b | c | d | e | f |
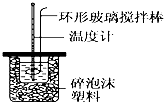
| �¶� ʵ����� | ��ʼ�¶�t1/�� | ��ֹ�� ��t2/�� | ƽ���¶Ȳ� ��t2-t1��/�� | ||
| H2SO4��Һ | NaOH��Һ | ƽ��ֵ | |||
| 1 | 26.2 | 26.0 | 26.1 | 30.1 | �����4.0�� �� |
| 2 | 27.0 | 27.4 | 27.2 | 33.3 | |
| 3 | 25.9 | 25.9 | 25.9 | 29.8 | |
| 4 | 26.4 | 26.2 | 26.3 | 30.4 | |
���� I����1������ʵ������245mL����ƿ����Ӧѡ��250mL������ƿ�������ó�250mL����Һ������m=CVM������������������ƹ����������
��2����������Ҫ�ڳ���ƿ�г��������ݳ������������������õ��������ش�
II����1����������кͷ�Ӧ����1molҺ̬ˮʱ�ų�57.3kJ��������д�Ȼ�ѧ����ʽ��
��2�����¶Ȳ�ƽ��ֵ���ڸ����¶Ȳ�ĺͳ���4��
�ڸ���Q=m•c•��T���㣻
��a��ʵ��װ�ñ��¡�����Ч������ã�
b����ȡNaOH��Һ�����ʱ����Ҫ�Ͱ�Һ����ƽ��
c�������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�Ҫ���¶ȼƻ���ֱ�ٲⶨH2SO4��Һ���¶ȣ�
��3��ϡ��ǿ����ǿ������1molH2O�ų�������Ϊ�к��ȣ�ע��������ʵĵ������Ⱥ�Ũ����ϡ�ͷ��������
��� �⣺I����1������ʵ������245mL����ƿ����Ӧѡ��250mL������ƿ�������ó�250mL����Һ��������������ƹ��������m=CVM=0.5mol/L��0.25L��40g/mol=5.0g���ʴ�Ϊ��250��5.0��
��2����������Ҫ�ڳ���ƿ����С�ձ��г������������������������õ���������ƽ���ձ���ҩ�ף��ʴ�Ϊ��a b e��
II����1��ϡǿ�ᡢϡǿ�Ӧ����1molˮʱ�ų�57.3kJ��������ϡ�������������ϡ��Һ�ֱ���ǿ�ᡢǿ���Ӧ���Ȼ�ѧ����ʽΪ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3 kJ/mol��
�ʴ�Ϊ��NaOH��aq��+$\frac{1}{2}$H2SO4��aq���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-57.3 kJ/mol��
��2�����¶Ȳ�ƽ��ֵ=$\frac{�Ĵ������У���ֹ�¶�-��ʼ�¶ȣ��ĺ�}{4}$�����ǵ�2������������������ɾ����
�¶Ȳ�ƽ��ֵ$\frac{��30.1-26.1��+��29.8-25.9��+��30.4-26.3��}{3}$=4.0��C���ʴ�Ϊ��4.0��
��50mL0.50mol/L����������30mL0.50mol/L������Һ�����кͷ�Ӧ����ˮ�����ʵ���Ϊ0.05L��0.50mol/L=0.025mol����Һ������Ϊ��80ml��1g/ml=80g���¶ȱ仯��ֵΪ��T=4�棬������0.025molˮ�ų�������ΪQ=m•c•��T=80g��4.18J/��g•�棩��4.0��=1337.6J����1.3376KJ������ʵ���õ��к��ȡ�H=-$\frac{1.3376KJ}{0.025mol}$=-53.5 kJ/mol��
�ʴ�Ϊ��-53.5kJ/mol��
��a��ʵ��װ�ñ��¡�����Ч������ã�����Ӱ��ʵ��������a��ȷ��
b����ȡNaOH��Һ�����ʱ���Ӷ������ᵼ�������������������ƫ�ų�������ƫ�ߣ������57.3kJ/mol����b����
c������һ�ο��ٽ�NaOH��Һ����ʢ�������С�ձ��У��������ֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У���c��ȷ��
d�����¶ȼƲⶨNaOH��Һ��ʼ�¶Ⱥ�Ҫ���¶ȼƻ���ֱ�ٲⶨH2SO4��Һ���¶ȣ���d��ȷ��
�ʴ�Ϊ��acd��
��3��ǿ����ǿ���ϡ��Һ�����кͷ�Ӧ����ЧӦ��H+��aq��+OH-��aq��=H2O��H=һ57.3kJ/mol��
���Ȼ�ѧ����ʽHNO3��aq��+NaOH��aq���TNaNO3��aq��+H2O��l����H=-Q3kJ•mol-1 �е�Q3=57.3kJ��
�����ڴ�����������ʣ��������ȣ����Ȼ�ѧ����ʽCH3COOH��aq��+NaOH��aq���TCH3COONa��aq��+H2O��l����H=-Q1 kJ•mol-1
�е�Q1��57.3KJ��
��Ũ����ϡ�ͷ��ȣ����Ȼ�ѧ����ʽ$\frac{1}{2}$H2SO4��Ũ��+NaOH��aq���T$\frac{1}{2}$Na2SO4��aq��+H2O��l����H=-Q2 kJ•mol-1
�е�Q2��57.3KJ��
�ʷų�������ΪQ2��Q3��Q1����ѡA��
���� ���⿼���Ȼ�ѧ����ʽ�Լ���Ӧ�ȵļ��㣬��Ŀ�Ѷȴ�ע�������к��ȵĸ�������Ȼ�ѧ����ʽ����д�������Լ��ⶨ��Ӧ�ȵ��������⣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʵ���� | c��NaOH��/mol•L-1 | c��HA��/mol•L-1 | �����Һ��pH |
| �� | 0.2 | 0.2 | pH=a |
| �� | 0.4 | 0.4 | pH=9 |
| �� | 0.4 | c1 | pH=7 |
| �� | 0.2 | 0.4 | pH��7 |
| A�� | ����������Һ��c��OH-��-c��HA��=10-9mol•L-1 | |
| B�� | c1��0.4 | |
| C�� | a��9 | |
| D�� | �ڶ�����Һ��c��Na+����c��A-����c��H+����c��OH-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | �٢ڢ� | B�� | �٢� | C�� | �٢ۢ� | D�� | �٢ڢۢ� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

 X�Ļ�ѧʽΪFe3C��P������Ļ�ѧʽΪCO2��NO2��
X�Ļ�ѧʽΪFe3C��P������Ļ�ѧʽΪCO2��NO2���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=1����Һ�У�Na+��ClO?��SO42?��I? | |
| B�� | c��OH-��=1��10-13mol/L����Һ�У�Mg2+��Cu2+��SO42-��NO3- | |
| C�� | ������Һ�У�Fe3+��K+��Cl?��SO42? | |
| D�� | ��ɫ��Һ�У�Fe2+��NH4+��SCN-��SO42- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 A��B��C��D��E����Ԫ�����ڱ���ǰ20��Ԫ�أ���ԭ��������������B��C��Dͬ���ڣ�A��Dͬ���壬E������Ԫ�ؼȲ���ͬ����Ҳ����ͬ���壮B��C��D������������Ӧˮ������ܻ������Ӧ�����κ�ˮ��A��E���γ����ӻ�����侧���ṹ��ͼ��ʾ��
A��B��C��D��E����Ԫ�����ڱ���ǰ20��Ԫ�أ���ԭ��������������B��C��Dͬ���ڣ�A��Dͬ���壬E������Ԫ�ؼȲ���ͬ����Ҳ����ͬ���壮B��C��D������������Ӧˮ������ܻ������Ӧ�����κ�ˮ��A��E���γ����ӻ�����侧���ṹ��ͼ��ʾ�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ԭ�����ֱ�Ӱѻ�ѧ��ת��Ϊ���ܵ�װ�ã�
ԭ�����ֱ�Ӱѻ�ѧ��ת��Ϊ���ܵ�װ�ã��鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com