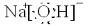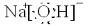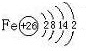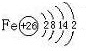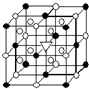
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa
3AlF
6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
AlF6-
AlF6-
��
Na+
Na+
��������������Ĵ�������������
AlF6-
AlF6-
������ʯ�ڻ��������е���;
������������ۼ�
������������ۼ�
��
��2��H
2S��H
2O
2����Ҫ�������ʱȽ����£�
|
�۵�/K |
�е�/K |
��״��ʱ��ˮ�е��ܽ�� |
| H2S |
187 |
202 |
2.6 |
| H2O2 |
272 |
423 |
������Ȼ��� |
H
2S��H
2O
2����Է�������������ͬ����������������ʲ������Ҫԭ��
H2O2���Ӽ�����������ˮ���ӿ��γ����
H2O2���Ӽ�����������ˮ���ӿ��γ����
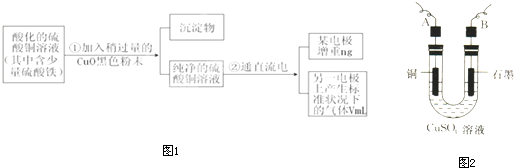
��3����ʢ������ͭˮ��Һ���Թ�����백ˮ�������γ�����������Ӱ�ˮ���������ܽ⣬�õ�����ɫ������Һ�������뼫�Խ�С���ܼ������Ҵ���������������ɫ�ľ��壮д��ͭԭ�Ӽ۵��Ӳ�ĵ����Ų�ʽ
3d104s1
3d104s1
����ͭͬһ���ڵĸ���Ԫ�صĻ�̬ԭ����������������ͭԭ����ͬ��Ԫ����
Cr
Cr
����Ԫ�ط��ţ���ʵ��ʱ�γɵ�����ɫ��Һ�е��������ڴ��ڵ�ȫ����ѧ��������
���ۼ�����λ��
���ۼ�����λ��
��ʵ������м���C
2H
5OH��ɹ۲쵽��������ɫCu��NH
3��
4SO
4?H
2O���壮ʵ��������C
2H
5OH��������
����Cu��NH3��4SO4?H2O���ܽ��
����Cu��NH3��4SO4?H2O���ܽ��
��
B���ú�������������ͭ��ȡ�Ȼ�ͭ���壨CuCl
2?xH
2O���������²�����
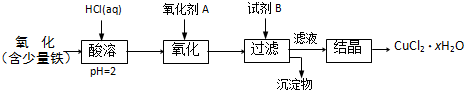
��֪����pHΪ4��5ʱ��Fe
3+������ȫˮ���������Cu
2+ȴ��ˮ�⣮
��1���������ܹ����з�����Ӧ�����ӷ���ʽ�У�
Fe+2H+=Fe2++H2����CuO+2H+=Cu2++H2O
Fe+2H+=Fe2++H2����CuO+2H+=Cu2++H2O
��2��������A��ѡ��
��
��
�����ţ���ͬ��
��Cl
2 ��KMnO
4 ��HNO
3��3��Ҫ�õ��ϴ��IJ�Ʒ���Լ�B��ѡ��
��
��
��NaOH ��FeO ��CuO
��4���Լ�B��������
�٢�
�٢�
�������Һ��pH �ڽ�����Һ��pH ��ʹFe
3+��ȫ���� ��ʹCu
2+��ȫ����
��5������Һ�����ᾧ�õ��Ȼ�ͭ����ķ�����
�ڢܢ�
�ڢܢ�
����ʵ���Ⱥ�˳�����ţ�
�ٹ��� ������Ũ�� ���������� ����ȴ
��6��Ϊ�˲ⶨ�Ƶõ��Ȼ�ͭ���壨CuCl
2?xH
2O����xֵ��ij��ȤС�����������ʵ�鷽����
����һ����ȡm g�����������������ټ���Ϊֹ����ȴ������������ˮCuCl
2������Ϊn g��
����������ȡm g���塢������������������Һ�����ˡ�����ϴ�Ӻ���С��������������ټ���Ϊֹ����ȴ���������ù��������Ϊn g��
��������������ʵ�鷽����������ȷ�ķ�����
��
��
���ݴ˼����x=
���ú�m��n�Ĵ���ʽ��ʾ����






 A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������
A����1����ͼ��ʾΪ����ʯ����ѧʽΪNa3AlF6���ľ�����ͼ�С�λ�ڴ������嶥������ģ���λ�ڴ��������12������е��8��С����������ģ���ͼ�С��е�һ�֣�ͼ�С�ֱ�ָ����������