
| K | Ca | Sc | Ti | V | Cr | Mn | Fe | Co | Ni | Cu | Zn | Ga | Ge |
·ÖĪö £Ø1£©4s¹ģµĄÉĻÖ»ÓŠ1øöµē×ÓµÄŌŖĖŲµÄĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ4s1”¢3d54s1”¢3d104s1£»
£Ø2£©øł¾ŻÄÜĮæ×īµĶŌĄķŹéŠ“Cr3+µÄµē×ÓÅŲ¼Ź½£»
sĒųµÄŌŖĖŲĪŖµŚIA”¢IIA×åŌŖĖŲ£»µŚIB”¢IIB×åĪŖdsĒų£¬ļēĻµŌŖĖŲ”¢ļ¹ĻµŌŖĖŲĪŖfĒų£¬¢óB”«¢÷B×å£ØļēĻµŌŖĖŲ”¢ļ¹ĻµŌŖĖŲ²śĪļ£©”¢µŚ¢ų×åĪŖdĒųŌŖĖŲ£¬¢óA”«¢÷A×唢Įć×åĪŖpĒų£»
£Ø3£©ø÷¹ģµĄ“¦ÓŚČ«Āś”¢°ėĀś”¢Č«æÕĪŖĪȶØדĢ¬£»
£Ø4£©°“ÕÕ“ÓµŚŅ»µ½µŚĖÄÖÜĘŚµÄĖ³ŠņŹéŠ“·ūŗĻĢõ¼žµÄŌ×ÓŗĖĶāµē×ÓÅŲ¼Ź½£»
£Ø5£©CaŌŖĖŲŌ×Ó4sÄܼ¶ĪŖČ«ĀśĪȶØדĢ¬£¬ÄÜĮæ½ĻµĶ£®
½ā“š ½ā£ŗ£Ø1£©4s¹ģµĄÉĻÖ»ÓŠ1øöµē×ÓµÄŌŖĖŲµÄĶāĪ§µē×ÓÅŲ¼Ź½ĪŖ4s1”¢3d54s1”¢3d104s1£¬·Ö±šĪŖ¼Ų”¢øõ”¢Ķ£¬
¹Ź“š°øĪŖ£ŗ¼Ų”¢øõ”¢Ķ£»
£Ø2£©Cr3+µÄµē×ÓÅŲ¼Ź½ĪŖ£ŗ[Ar]3d3 £¬Ga“¦ÓŚµŚĖÄÖÜĘŚ¢óA×壬ŹōÓŚpĒųŌŖĖŲ£¬
¹Ź“š°øĪŖ£ŗ[Ar]3d3 £»p£»
£Ø3£©Fe3+µÄ3d¹ģµĄĪŖ°ė³äĀś×“Ģ¬£¬»ÆѧŠŌÖŹ±ČFe2+ĪČ¶Ø£¬
¹Ź“š°øĪŖ£ŗFe3+µÄ3d¹ģµĄĪŖ°ė³äĀś×“Ģ¬£»
£Ø4£©µŚŅ»ÖÜĘŚÖŠ£¬ÓŠ1øöĪ“³É¶Ōµē×Ó£¬Ęäµē×ÓÅŲ¼Ź½ĪŖ1s1£»µŚ¶žÖÜĘŚÖŠ£¬Ī“³É¶Ōµē×ÓŹĒ2·Ö±šĪŖ£ŗ1s22s22p2”¢1s22s22p4£»µŚČżÖÜĘŚÖŠĪ“³É¶Ōµē×ÓŹĒ3øöµÄŹĒ1s22s22p63s23p3£»µŚĖÄÖÜĘŚÖŠĪ“³É¶Ōµē×ÓŹĒ4øöµÄŹĒ1s22s22p63s23p63d64s2£¬
¹Ź“š°øĪŖ£ŗ5£»
£Ø5£©CaŌŖĖŲŌ×Ó4sÄܼ¶ĪŖČ«ĀśĪȶØדĢ¬£¬ÄÜĮæ½ĻµĶ£¬µŚŅ»µēĄėÄÜøßÓŚĶ¬ÖÜĘŚĻąĮŚŌŖĖŲµÄ£¬¹ŹµŚŅ»µēĄėÄÜI1£ØK£©£¼I1£ØCa£©£¬
¹Ź“š°øĪŖ£ŗ£¼£®
µćĘĄ ±¾ĢāŹĒ¶ŌĪļÖŹ½į¹¹ÓėŠŌÖŹµÄ漲飬Éę¼°ŗĖĶāµē×ÓÅŲ¼”¢ŌŖĖŲÖÜĘŚ±ķ”¢µēĄėÄÜµČ£¬×¢ŅāĄķ½āµŚŅ»µēĄėÄÜŅģ³£Ēéæö”¢ÕĘĪÕŗĖĶāµē×ÓÅŲ¼¹ęĀÉ£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
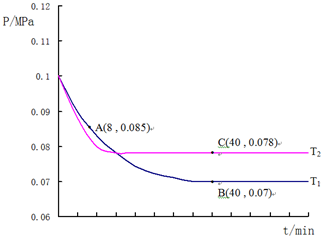
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| ±ąŗÅ | ¢Ł | ¢Ś | ¢Ū | ¢Ü |
| Ļ”ĻõĖįµÄĢå»ż/mL | 100 | 200 | 300 | 400 |
| Ź£Óą½šŹōµÄÖŹĮæ/g | 18.0 | 9.6 | 0 | 0 |
| NO µÄĢå»ż/mL | 2240 | 4480 | 6720 | V |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĶʶĻĢā
| ŌŖĖŲ“śŗÅ | A | B | C | D | E | F | G |
| Ō×Ó°ė¾¶/nm | 0.037 | 0.16 | 0.143 | 0.102 | 0.099 | 0.074 | 0.075 |
| Ö÷ŅŖ»ÆŗĻ¼Ū | +1 | +2 | +3 | +6”¢-2 | -1 | -2 | +5”¢-3 |
 £»
£» NH4++NH2-£®
NH4++NH2-£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ĒāŃõ»Æ±µČÜŅŗÓėĮņĖį»ģŗĻ£ŗBa2++2OH-+2H++SO42-ØTBaSO4”ż+2H2O | |
| B£® | Ģś·ŪÓėĻõĖįŅųČÜŅŗ·“Ó¦£ŗFe+2Ag+ØTFe2++2Ag | |
| C£® | ĀČ»ÆĀĮČÜŅŗÓė¹żĮæµÄ°±Ė®·“Ó¦£ŗAl3++4NH3•H2OØTAlO2-+4NH4++2H2O | |
| D£® | ĀČĘųĶØČėĄäµÄĒāŃõ»ÆÄĘČÜŅŗÖŠ£ŗCl2+2OH-ØTCl-+ClO-+H2O |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ¼ģ²é²”ČĖĪø²”ĖłÓƵıµ²Ķ£¬Ö»ÄÜÓĆBaSO4£¬²»æÉŅŌÓĆBaCO3 | |
| B£® | ĪŖĮĖŹ¹ĀųĶ·”¢Ņų¶śµČ½ą°×”¢ĀōĻąŗĆ£¬æÉŅŌÓƵćČ¼Įņ»Ē·ØѬÕōĖüĆĒ | |
| C£® | æÉŅŌÓĆSO2Ą“ĘÆ°×Ö½½¬”¢Ć«”¢Ė攢²ŻĆ±±čµČ | |
| D£® | ”°Įņ»Ē”±ĪĀČŖæÉŅŌŅ½ÖĪʤ·ō²” |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | ·“Ó¦¢ŚÖŠµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£¬Ōņ”÷H2=E1-E3 | |
| B£® | N2µÄČ¼ÉÕČČĪŖ180 kJ•mol-1 | |
| C£® | ÓÉ·“Ó¦¢ŚÖŖŌŚĪĀ¶ČŅ»¶ØµÄĢõ¼žĻĀ£¬ŌŚŅ»ŗćČŻĆܱÕČŻĘ÷ÖŠĶØČė1molN2ŗĶ3molH2£¬·“Ó¦ŗó·Å³öµÄČČĮæĪŖQ1kJ£¬ŌņQ1=92.4 | |
| D£® | °±µÄ“ß»ÆŃõ»Æ·“Ó¦ĪŖ4NH3£Øg£©+5O2£Øg£©=4NO£Øg£©+6H2O£Øg£©”÷H=-906 kJ•mol-1 |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com