
 ¹ŽÖŠŹÕ¼ÆĀśCO2£¬Č»ŗóµ¹Čė10mLÅØNaO
¹ŽÖŠŹÕ¼ÆĀśCO2£¬Č»ŗóµ¹Čė10mLÅØNaO HČÜŅŗ£¬ŃøĖŁĆÜ·āŅץ¹ŽæŚ£¬æÉŅŌ¹Ū²ģµ½Ņץ¹ŽĶ»Č»±ä±ńĮĖ£¬ŌŅņŹĒ £ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß”£
HČÜŅŗ£¬ŃøĖŁĆÜ·āŅץ¹ŽæŚ£¬æÉŅŌ¹Ū²ģµ½Ņץ¹ŽĶ»Č»±ä±ńĮĖ£¬ŌŅņŹĒ £ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ £ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß”£ £ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß”£
£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß£ß”£ ĆūŠ£æĪĢĆĻµĮŠ“š°ø
ĆūŠ£æĪĢĆĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®ĀĮÖĘĘ·ŌŚÉś»īÖŠ±»¹ć·ŗŹ¹ÓĆ£¬ĖµĆ÷ĀĮŹĒŅ»ÖÖ²»»īĘĆµÄ½šŹō |
| B£®Ńõ»ÆĀĮµÄČŪµćŗÜøߣ¬ŹĒŅ»ÖÖ½ĻŗƵÄÄĶ»š²ÄĮĻ |
| C£®Al(OH)3¼īŠŌŗÜĒ棬¹ŹæÉÓĆÓŚÖĪĮĘĪøĖį¹ż¶ą |
| D£®ĀĮŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®1£ŗ1 | B£®2£ŗ3 | C£®3£ŗ2 | D£®1£ŗ6 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®1”Ć3 | B£®1”Ć2 | C£®3”Ć1 | D£®3”Ć2 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
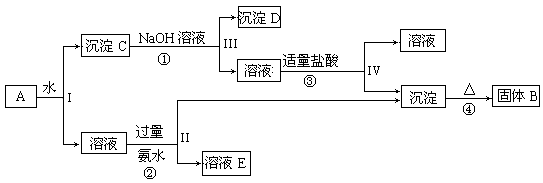
| A£®Ō»ģŗĻČÜŅŗÖŠµÄCO32-ÓėAlO2-µÄĪļÖŹµÄĮæÖ®±ČĪŖ1£ŗ2 |
| B£®V1£ŗV2=1£ŗ5 |
| C£®MµćŹ±Éś³ÉµÄCO2ĪŖ0£®05mol |
| D£®aĒśĻß±ķŹ¾µÄĄė×Ó·½³ĢŹ½ĪŖ£ŗAlO2-+H++H2O=Al£ØOH£©3”ż |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
| A£®100mL | B£®150mL | C£®200mL | D£®300mL |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
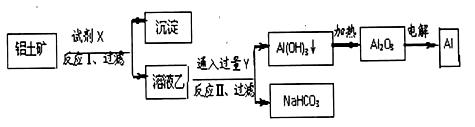
| A£®ŹŌ¼ĮXĪŖĒāŃõ»ÆÄĘČÜŅŗ£¬ŹŌ¼ĮYĪŖCO2 |
| B£®½įŗĻÖŹ×Ó£ØH+£©µÄÄÜĮ¦ÄĻĒæµ½ČõµÄĖ³ŠņŹĒCO32->AlO2->OH |
| C£®·“Ó¦¢ņÖŠÉś³ÉAl£ØOH£©3µÄ·“Ó¦ĪŖCO2+AlO2-+2H2O=Al£ØOH£©3”ż+HCO3- |
| D£®Al2O3ČŪµćŗÜøߣ¬µē½āŹ±ĻņAl2O3ÖŠ¼ÓČė±ł¾§ŹÆ£¬Ź¹Al2O3µÄČŪµć½µµĶ£¬“Ó¶ų¼õÉŁŅ±Į¶¹ż³ĢÖŠÄÜĮæĻūŗÄ”£ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com