

·ÖĪö £Ø1£©ÓūÅäÖĘ230mL 0.100mol•L-1 Na2CO3ČÜŅŗ£¬ŠčŅŖŃ”Ōń250mLČŻĮæĘ棬Źµ¼ŹÅäÖĘ250mLČÜŅŗ£¬ŅĄ¾Żm=CVM¼ĘĖćČÜÖŹµÄÖŹĮ棻ČōĖłČ”µÄ¾§ĢåŅŃ¾ÓŠŅ»²æ·ÖŹ§Č„ĮĖ½į¾§Ė®£¬µ¼ÖĀ³ĘČ”µÄČÜÖŹµÄĪļÖŹµÄĮæĘ«“ó£¬ŅĄ¾ŻC=$\frac{n}{V}$ÅŠ¶ĻĪó²ī£»
£Ø2£©ŅĄ¾ŻÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗÖŠŅĘŅŗ”¢¶ØČŻµÄÕżČ·²Ł×÷£¬·ÖĪö²Ł×÷¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»żµÄÓ°Ļģ£¬ŅĄ¾ŻC=$\frac{n}{V}$ÅŠ¶ĻĪó²ī£¬¾Ż“Ė½ā“š£»
£Ø3£©ŅĄ¾ŻÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½ÖčÅÅŠņ£»
£Ø4£©ČŻĮæĘæĪŖÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗµÄ¼ĘĮæČŻĘ÷£¬Ö»ÄÜŌŚ³£ĪĀĻĀŹ¹ÓĆ£¬²»Ķ¬µÄ¹ęøńµÄČŻĮæĘæÅäÖʵÄČÜŅŗµÄĢå»ż²»Ķ¬£¬ĒŅŌŚŹ¹ÓĆĒ°Ó¦¼ģ²éŹĒ·ńĀ©Ņŗ£®
½ā“š ½ā£ŗ£Ø1£©ÓūÅäÖĘ230mL 0.100mol•L-1 Na2CO3ČÜŅŗ£¬ŠčŅŖŃ”Ōń250mLČŻĮæĘ棬Źµ¼ŹÅäÖĘ250mLČÜŅŗ£¬ŠčŅŖNa2CO3£®10H2OµÄÖŹĮæm=CVM=0.100mol/L”Į0.25L
”Į286g/mol=7.2g£»ČōĖłČ”µÄ¾§ĢåŅŃ¾ÓŠŅ»²æ·ÖŹ§Č„ĮĖ½į¾§Ė®£¬µ¼ÖĀ³ĘČ”µÄČÜÖŹµÄĪļÖŹµÄĮæĘ«“ó£¬ŅĄ¾ŻC=$\frac{n}{V}$æÉÖŖ£¬ČÜŅŗÅضČĘ«“ó£»
¹Ź“š°øĪŖ£ŗ250mL£»7.2£» Ę«“ó£»
£Ø2£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗÖŠŅĘŅŗ²Ł×÷ÖŠ²£Į§°ōµÄ×÷ÓĆŹĒŅżĮ÷£»²½ÖčAĶس£³ĘĪŖ¶ØČŻ£¬“ĖŹ±Ó¦×¢ŅāŹÓĻßÓė°¼ŅŗĆę£¬æĢ¶ČĻßĖ®Ę½ĻąĒŠ£¬Čē¹ūø©ŹÓæĢ¶ČĻߣ¬µ¼ÖĀČÜŅŗĢå»żĘ«Š”£¬ČÜŅŗÅضČĘ«“ó£»²½Öč½įŹųŗó·¢ĻÖŅŗĆęµĶÓŚæĢ¶ČĻߣ¬ŹōÓŚÕż³£²Ł×÷£¬¶ŌČÜÖŹµÄĪļÖŹµÄĮæŗĶČÜŅŗĢå»ż¶¼²»²śÉśÓ°ĻģČÜŅŗÅØ¶Č²»±ä£»
¹Ź“š°øĪŖ£ŗŅżĮ÷£¬¶ØČŻ£¬°¼ŅŗĆę£¬æĢ¶ČĻߣ¬Ę«“ó£¬ĪŽÓ°Ļģ£»
£Ø3£©ÅäÖĘŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗŅ»°ć²½Öč£ŗ¼ĘĖć”¢³ĘĮ攢Čܽā”¢ŅĘŅŗ”¢Ļ“µÓ”¢¶ØČŻ”¢Ņ”ŌČ£¬ĖłŅŌÕżČ·µÄĖ³ŠņĪŖ£ŗC”¢B”¢D”¢F”¢A”¢E£»
¹Ź“š°øĪŖ£ŗC”¢B”¢D”¢F”¢A”¢E£»
£Ø4£©A£®ČŻĮæĘæÅäÖĘČÜŅŗŹ±£¬ŠčŅŖ¶ØČŻĖłŅŌŠčŅŖ¼ÓČėÕōĮóĖ®£¬Ź¹ÓĆĒ°²»ŠėøÉŌļ£¬¹ŹA“ķĪó£»
B£®ČŻĮæĘæŹ¹ÓĆĒ°ĻČ¼ģ²éĘæČū²»Ā©Ė®£¬¹ŹBÕżČ·£»
C£®ČŻĮæĘæ²»ÄÜÓĆĄ“³¤ĘŚ“ę“¢ČÜŅŗ£¬¹ŹC“ķĪó£»
D£®ČŻĮæĘæĪŖ¾«ĆÜŅĒĘ÷£¬Ö»ÓŠŌŚŹŅĪĀĻĀĢå»ż²Å¾«Č·£¬¹ŹDÕżČ·£»
E£®ČŻĮæĘæÓĆÓŚ×¼Č·ÅäÖĘŅ»¶ØĢå»żµÄŅ»¶ØĪļÖŹµÄĮæÅØ¶ČµÄČÜŅŗ£¬²»ÄÜÅäÖĘČĪŅāĢå»żČÜŅŗ£¬¹ŹE“ķĪó£»
¹ŹŃ”£ŗBD£®
µćĘĄ ±¾Ģāæ¼²éĮĖŅ»¶ØĪļÖŹµÄĮæÅضČČÜŅŗµÄÅäÖĘ£¬Ć÷Č·ÅäÖĘŌĄķŗĶ²½ÖčŹĒ½āĢā¹Ų¼ü£¬×¢ŅāČŻĮæĘæµÄŃ”ŌńŗĶŹ¹ÓĆ·½·Ø£¬ĢāÄæÄŃ¶Č²»“ó£®


 ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø
ĢģĢģĻņÉĻŅ»±¾ŗĆ¾ķĻµĮŠ“š°ø Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
Š”ѧɜ10·ÖÖÓÓ¦ÓĆĢāĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ¶ąŃ”Ģā
| A£® | ŌŚ³£ĪĀ³£Ń¹ĻĀ£¬11.2L N2ŗ¬ÓŠµÄ·Ö×ÓŹżĪŖNA | |
| B£® | ±ź×¼×“æöĻĀ£¬18g H2OĖłÕ¼µÄĢå»żŌ¼ŹĒ22.4L | |
| C£® | 32gO2ŌŚ±ź×¼×“æöĻĀĖłÕ¼µÄĢå»żŌ¼ĪŖ22.4L | |
| D£® | 1mol NeÖŠŌ¼ŗ¬ÓŠ6.02”Į1024øöµē×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | N4ŗĶN2ŹĒ»„ĪŖĶ¬Ī»ĖŲ | |
| B£® | N4µÄĦ¶ūÖŹĮæŹĒ56g | |
| C£® | ĻąĶ¬ÖŹĮæµÄN4ŗĶN2Ėłŗ¬Ō×ÓøöŹż±ČĪŖ2£ŗ1 | |
| D£® | ĆæøöN4·Ö×Óŗ¬ÓŠ28øöµē×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| ²Ł×÷ | ĻÖĻó | ½įĀŪ | |
| A | µĪ¼ÓĻ”ŃĪĖį | ÓŠĘųÅŻ²śÉś | ŌČÜŅŗÖŠÓŠCO32- |
| B | µĪ¼ÓŃĪĖįĖį»ÆµÄ BaCl2ČÜŅŗ | Éś³É°×É«³Įµķ | ŌČÜŅŗÖŠÓŠAg+ »ņSO42- |
| C | ÓĆ½ą¾»²¬ĖæÕŗČ”ČÜŅŗ ½ųŠŠ×ĘÉÕ | »šŃę³Ź»ĘÉ« | ŌČÜŅŗÖŠÓŠNa+”¢ ĪŽK+ |
| D | µĪ¼ÓĻ”NaOHČÜŅŗ£¬½«ŹŖ ČóŗģÉ«ŹÆČļŹŌÖ½ÖĆÓŚŹŌ¹ÜæŚ | ŹŌÖ½²»±äĄ¶ | ŌČÜŅŗÖŠĪŽNH4+ |
| A£® | A | B£® | B | C£® | C | D£® | D |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

| ĪļÖŹĄą±š | Ėį | ¼ī | ŃĪ | Ńõ»ÆĪļ | Ēā»ÆĪļ |
| »ÆѧŹ½ | ¢ŁH2SO4 ¢Ś | ¢ŪNaOH ¢Ü | ¢ŻNa2SO4 ¢Ž | ¢ßSO2 ¢ąSO3 | ¢įNH3 ¢ā |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

 £»
£»
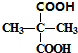 +2C2H5OH$”ś_{”÷}^{ÅØĮņĖį}$
+2C2H5OH$”ś_{”÷}^{ÅØĮņĖį}$ +2H2O£®
+2H2O£® Óė×ćĮæNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ
Óė×ćĮæNaOHČÜŅŗ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ £®
£® ”¢
”¢ £®
£®²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā

²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com