
X��Y��Z��W��R��5�ֶ�����Ԫ�أ���ԭ��������������X�����ڱ���ԭ�Ӱ뾶��С��Ԫ�أ�Yԭ�������������Ǵ�����������3����Z��W��R����ͬһ���ڣ�R��Y����ͬһ�壬Z��Wԭ�ӵĺ��������֮����Y��Rԭ�ӵĺ��������֮����ȡ�����˵����ȷ���� �� ��
A. Ԫ��Y��Z��W������ͬ���Ӳ�ṹ�����ӣ���뾶��������
B. Ԫ��X������Ԫ��Y�γɻ�����X2Y2
C. Ԫ��Y��R�ֱ���Ԫ��X�γɵ����������ȶ��ԣ�XmY>XmR
D. Ԫ��W��R������������ˮ���ﶼ��ǿ��
 ��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
��ĩ���䵥Ԫ�����ิϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2017���㽭ʡ�����и�����ѧ����Ӧ�Կ��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ���ǣ� ��
A����������������ˮ������ B�����������ǰ뵼�����
C�������������������ھ�ˮ D���������������Ư��ֽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ�ڵ�һ��������ѧ�Ծ��������棩 ���ͣ������
ij��ͬѧ������ʵ��̽��Na2CO3��NaHCO3�����ʡ��ش��������⣺
��1������ͬѧ�ֱ�ȡ1.0g Na2CO3��NaHCO3���壬���μӼ���ˮ������ʢNa2CO3���Թ��¶���������ʢNaHCO3���Թ��¶��½���ԭ����______________��
��2������ͬѧ�ֱ�ȡ0.1mol/L Na2CO3��0.1mol/LNaHCO3����Һ����0.1mol/LHCl��Һ�ζ���pH�仯����������Ĺ�ϵ��ͼ��ʾ��
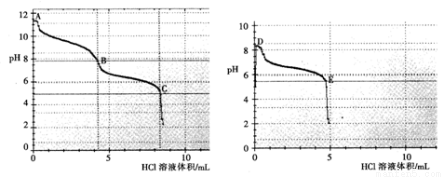
̼���������ᷴӦpH������ͻ�䣬�ﵽ���η�Ӧ�յ㣬�ֱ���__________����A��B��C��D��E����
B�����Ҫ������_____________���û�ѧʽ��ʾ����
�� B��__________������ڡ���С�ڡ�) D���pH����Ҫԭ����________________��
��3������ͬѧԤ��NaHCO3�����ԣ�������Һ����þ�ۣ����������ݺͳ������ɣ�д����ѧ����ʽ��_________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�콭��ʡ������ѧ�ڵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
���з�Ӧ���������ӷ���ʽ��H++OH-=H2O����ʾ����
A.H2SO4��Һ��NaOH��Һ��� B.HCl����ͨ��Ca(OH)2��Һ��
C.HNO3��Һ��KOH��Һ��� D.NH4HSO4��Һ��NaOH��Һ���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ�����ϵڶ���ģ�⻯ѧ�Ծ��������棩 ���ͣ�ʵ����
����þ[Mg(ClO3)2]����������������ݼ��ȣ�ʵ�����Ʊ���Mg(ClO3)2��6H2O���������£�

��֪����±����Ҫ�ɷ�ΪMgCl2��6H2O������MgSO4��FeCl2�����ʡ�
�ڼ��ֻ�������ܽ��(S)���¶�(T)�仯������ͼ��
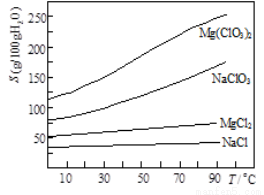
��1����MgO�����������������Ҫ�ɷ�Ϊ _____��
��2������BaCl2��Ŀ���dz�ȥSO42������μ���SO42���ѳ�����ȫ�� ___��
��3������NaClO3������Һ�ᷢ�����·�Ӧ��MgCl2��2NaClO3 Mg(ClO3)2��2NaCl���������ø÷�Ӧ�������ͼ����ȡMg(ClO3)2��6H2O��ʵ�鲽������Ϊ�� ��ȡ��������NaClO3������Һ��ַ�Ӧ���������ᾧ���� ���� ���ݹ��ˡ�ϴ�ӡ�
Mg(ClO3)2��2NaCl���������ø÷�Ӧ�������ͼ����ȡMg(ClO3)2��6H2O��ʵ�鲽������Ϊ�� ��ȡ��������NaClO3������Һ��ַ�Ӧ���������ᾧ���� ���� ���ݹ��ˡ�ϴ�ӡ�
��4������Ʒ����ˮϴ�ӣ�������ˮ�Ҵ���ϴ����ˮ�Ҵ���������___________________��
��Ʒ��Mg(ClO3)2��6H2O������Է�������Ϊ299�������IJⶨ��
����1��ȷ����3.50g��Ʒ�ܽⶨ�ݳ�100mL��Һ��
����2��ȡ10mL����ƿ�У�����10mLϡ�����20mL1.000mol/L��FeSO4��Һ���ȡ�
����3����ȴ�����£���0.100mol/LK2Cr2O7��Һ�ζ����յ㡣�˹����з�Ӧ�����ӷ���ʽΪ��
Cr2O72����6Fe2����14H�� 2Cr3����6Fe3����7H2O��
2Cr3����6Fe3����7H2O��
����4��������2��3�ظ����Σ�ƽ������K2Cr2O7��Һ15.00mL��
��5��д������2�з��������ӷ���ʽ_______________________��
��6��������Ʒ�ⶨ����Ҫ����Ƿ�©Һ��������_____________________��
����3�����ζ�ǰ���ñ�Һ��ϴ�ζ��ܣ��ᵼ�����ս��ƫ___________�����С������
��7����Ʒ��Mg(ClO3)2��6H2O����������Ϊ ______ ��(������������λС��)
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������9���¿���ѧ�Ծ��������棩 ���ͣ�ʵ����
��������п�������Ļ��Լ�����ǿ����������п��Ʒ�������������CuO��SiO2�����ʣ�Ϊԭ���Ʊ���������п��������������Ʒ�����������������£�

��֪��Zn(OH)2������NaOH��Һ��һЩ������������������ʽ��ȫ����ʱ��Һ��pH���±���

��1������A���õIJ����������ձ���©����______________��
��2�����ܽ⡱ǰ������п��Ʒ�����ϸ������Ŀ����____________________��
��3������������ɻ��յĽ�����_______________��
��4��������������ҺN�м���������H2O2��Һ���ټ�һ����ij�Լ�������ҺpH=5����Fe3+Ũ��Ϊ__________����֪Ksp[Fe(OH)3]=4.0��10-34��
��5����K3[Fe(CN)6]�����軯�أ���֤N��Fe2+��������___________��
��6�����������ijɷ�ΪZnCO3��2Zn(OH)2��H2O�������ա���450��500���½��У����ջ��ZnO�ķ�Ӧ�Ļ�ѧ����ʽΪ_____________________��
��7��ȡ20.00mL�˶�N����0.02mol/L��KMnO4��Һ���еζ�������KMnO4��Һ18.00mL������ҺN��Fe2+��Ũ��Ϊ__________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2017�����ʡ������9���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����˵������ȷ����( )
A��Ư������Һ�д���ƽ�⣺ClO-+H2O HClO+OH-��������NaOH�������Ư��Ч��
HClO+OH-��������NaOH�������Ư��Ч��
B�����ᱵ������ˮ�������ᱵ����ǿ�����
C���ζ�ǰ�ζ����������ݣ��յ����ʱ�����ݣ��������ƫ��
D�����ʵ���Ũ����ͬ��������Һ�����Ȼ�梨�������� �۴�����梨�̼��淋�pH����>��>��>��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�꼪��ʡ��һ���ڳ���ѧ���������棩 ���ͣ�ѡ����
����˵����ȷ����
A����������ͬ��CO��N2�����һ������
B �������ͬ��CO��N2������һ�����
�������ͬ��CO��N2������һ�����
C��������ͬ���ܶ���ͬ��CO��N2����������������ͬ
D��������ͬ���ܶȲ�ͬ��CO��N2������ԭ����Ŀһ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2016-2017ѧ�����ʡ��һ�ϵ�һ�ο��Ի�ѧ���������棩 ���ͣ�ѡ����
�������ܱ������У��ֱ����������ͬ�ļס����������壬�����������¶Ⱥ�ѹǿ����ͬ���Ҽ��ܶȴ����ҵ��ܶȣ�������˵����ȷ����
A���ķ��������ҵķ������� B�������ʵ������ҵ����ʵ���С
C������Ħ���������<�� D������Է����������ҵ���Է�������С
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com