
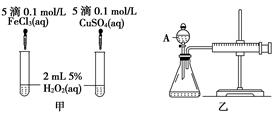
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��

(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6 mol X�����0.6 mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g)  C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯
B����������ܶȲ�����ʱ����仯
C��A���������ٸı�
D��ƽ���������ƽ����Է����������ٸı�
(1)��Ӧ�������ݿ�����������������ͬ���ų������ӵĸ���
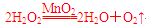
(2)��Һ©�����ռ�40mL��������Ҫ��ʱ�䡡�ر�A�ϵIJ�������������Ͳ�Ļ�������(���ƽ�)һ�κ��ɿ��������ܻص�ԭ����λ��˵������������(�����𰸺���Ҳ����)
(3)v(X)��v(W)��0.05 mol��L��1��min��1�� 0.25 mol/L��4��
(4)A
����������1�������IJ�ͬ������Ӧ���ʵIJ�ͬ�����Ը��ݷ�Ӧ�в������ݵĿ��������Է��������Ĵ�Ч����Ϊ����ʵ����пɱ��ԣ�Ӧ��ʹ��Һ�е�������Ҳ��ͬ�����������ų������Ӳ�ͬ������ɵĸ��š�
��2������۷���A�Ƿ�Һ©�����ռ���ͬ������������Ҫ��������ʱ��ij��̡�����װ�õ��Ҳ��Ǵ��л�������Ͳ�����Լ�����������ʱ�������ô�ͳ�ķ������飬��ȷ�ķ����ǹر�A�ϵIJ�������������Ͳ�Ļ�������(���ƽ�)һ�κ��ɿ��������ܻص�ԭ����λ��˵�����������ã�����©����
��3����Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)���������Z�����ʵ�����0.1 mol��(L��min)��2L��2min=0.4mol��ͬʱ����W��0.2mol����Ϊ���ʵı仯��֮������Ӧ�Ļ�ѧ������֮�ȣ�����n����4.����Ϊ���ʵ����ı仯������Ӧ�Ļ�ѧ�������dz����ȵģ���������֮��Ӧ������Ӧ�Ļ�ѧ������֮�ȣ�����X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ҳ��0.05 mol��(L��min)������0.2molW���ͱ�Ȼ����0.1molY����ʣ��Y��0.5mol������2 minĩʱY�����ʵ���Ũ��Ϊ0.25mol/L��
��4����Ϊ��Ӧǰ�����������Dz���ģ������ڷ�Ӧ������ѹǿʼ���Dz���ģ�A����ȷ���ܶ��ǻ�����������������ݻ��ı�ֵ���ݻ����䣬��������������DZ仯�ģ����Ե���������ܶȲ�����ʱ����仯ʱ������˵���ﵽƽ��״̬��A���������ٸı䣬˵�����淴Ӧ������ȣ����ѡ��C��ȷ����������ƽ����Է��������ǻ�����������ͻ���������ʵ����ı�ֵ������������ʵ������䣬�������DZ仯�ģ���˵�ƽ���������ƽ����Է����������ٸı�ʱ����˵���Ѿ��ﵽƽ��״̬��


 ȫ��������ϵ�д�
ȫ��������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6mol X�����0.6mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g) C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯
B����������ܶȲ�����ʱ����仯
C��A���������ٸı�
D��ƽ���������ƽ����Է����������ٸı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6mol X�����0.6mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g) C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯
B����������ܶȲ�����ʱ����仯
C��A���������ٸı�
D��ƽ���������ƽ����Է����������ٸı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012ѧ���Ĵ�ʡ��ɽ��ѧ��һ��ѧ�����п��Ի�ѧ�Ծ����������� ���ͣ�ʵ����
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6 mol X�����0.6 mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g)  C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
| A�������ڵ�ѹǿ����ʱ����仯 |
| B����������ܶȲ�����ʱ����仯 |
| C��A���������ٸı� |
| D��ƽ���������ƽ����Է����������ٸı� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013��ɽ��ʡ�߶���ѧ������ģ��ѧ���϶����Ի�ѧ�Ծ� ���ͣ�ʵ����
(10��)���о�֪Cu2����H2O2�ֽ�Ҳ���д����ã�Ϊ�Ƚ�Fe3����Cu2����H2O2�ֽ�Ĵ�Ч����ij�о�С���ͬѧ�ֱ��������ͼ�ס�����ʾ��ʵ�顣�ش�������⣺
(1) ���Է�������ͼ��ͨ���۲�____________________________________�����ԱȽϵó����ۡ���ͬѧ�����FeCl3��ΪFe2(SO4)3��Ϊ��������������____________________________________________________________________��
д��H2O2�ڶ������������·�����Ӧ�Ļ�ѧ����ʽ
____________________________________________________________________��
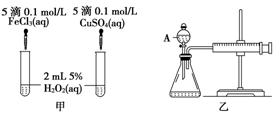
(2) ������������ͼ����ʾ��ʵ��ʱ��������40mL����Ϊ����������Ӱ��ʵ������ؾ��Ѻ��ԡ�ͼ������A������Ϊ________��ʵ������Ҫ������������___________________________________________________________��
�����װ�������Եķ�����
____________________________________________________________________��
(3) 0.6 mol X�����0.6 mol Y��������2 L�ܱ������У��������·�Ӧ��
2X(g)��Y(g)===nZ(g)��2W(g)�� 2 minĩ����0.2 mol W���������Z�����ʵ���Ũ�ȱ仯��ʾ�ķ�Ӧ����Ϊ0.1 mol��(L��min)����ǰ2 min�ڣ���X�����ʵ���Ũ�ȱ仯��ʾ��ƽ����Ӧ����Ϊ________��2 minĩʱY�����ʵ���Ũ��Ϊ________����ѧ����ʽ�У�Z�Ļ�ѧ������n��________��
(4) ��һ���¶��£���Ӧ��2A(s)��2B(g)  C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
C(g)��D(g)�ں��������н��У�����˵���÷�Ӧ�Ѿ��ﵽƽ�����________
A�������ڵ�ѹǿ����ʱ����仯
B����������ܶȲ�����ʱ����仯
C��A���������ٸı�
D��ƽ���������ƽ����Է����������ٸı�
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com