
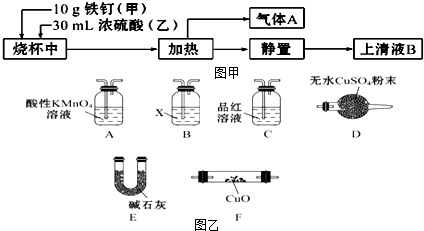
·ÖĪö £Ø1£©ĻČŌŚÉÕ±ÖŠ¼ÓĢś¶¤£¬ŌŁ¼ÓÅØĮņĖį£»
£Ø2£©Fe2+¾ßÓŠ»¹ŌŠŌ£¬ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ«£»
£Ø3£©Ģś¶¤ÖŠŗ¬ÓŠÉŁĮæµÄĢ¼ŌŖĖŲ£¬CÓėÅØĮņĖį·“Ӧɜ³É¶žŃõ»ÆĢ¼”¢¶žŃõ»ÆĮņŗĶĖ®£»
£Ø4£©¼ģŃéĘųĢåAÖŠŹĒ·ńŗ¬ÓŠH2ŗĶCO2£¬Ó¦øĆĻČ³żČ„¶žŃõ»ÆĮņ£¬Č»ŗóÓĆ³ĪĒåŹÆ»ŅĖ®¼ģŃ鶞Ńõ»ÆĢ¼£¬ŌŁøÉŌļ£¬Č»ŗóĶعżCuO£¬ÓėCuO·“Ӧɜ³ÉĖ®£¬ÓĆĪŽĖ®ĮņĖįĶ¼ģŃéĖ®µÄÉś³É£¬¼“æÉÖ¤Ć÷ÓŠĒāĘų£®
½ā“š ½ā£ŗ£Ø1£©ĻČŌŚÉÕ±ÖŠ¼ÓĢś¶¤£¬ŌŁ¼ÓÅØĮņĖį£¬Čē¹ūĻČ¼ÓČėÅØĮņĖį£¬ŌŁ¼ÓĢś¶¤£¬ŌņČŻŅ×½¦ĘšÅØĮņĖįÉĖČĖ£»
¹Ź“š°øĪŖ£ŗ¼×£»
£Ø2£©ÓÉÓŚĢś¶¤ÉśŠā£¬ÉĻĒåŅŗBÖŠæÉÄܼČŗ¬Fe3+£¬ÓÖŗ¬Fe2+£¬Fe2+¾ßÓŠ»¹ŌŠŌ£¬ÄÜŹ¹ĖįŠŌKMnO4ČÜŅŗĶŹÉ«£¬ĖłŅŌÓĆĖįŠŌKMnO4ČÜŅŗ¼ģŃéÓŠĪŽFe2+£»
¹Ź“š°øĪŖ£ŗd£»
£Ø3£©Ģś¶¤ÖŠŗ¬ÓŠÉŁĮæµÄĢ¼ŌŖĖŲ£¬CÓėÅØĮņĖį·“Ӧɜ³É¶žŃõ»ÆĢ¼”¢¶žŃõ»ÆĮņŗĶĖ®£¬Ęä·“Ó¦µÄ·½³ĢŹ½ĪŖ£ŗC+2H2SO4£ØÅØ£©$\frac{\underline{\;\;”÷\;\;}}{\;}$2SO2”ü+CO2”ü+2H2O£»
¹Ź“š°øĪŖ£ŗC+2H2SO4£ØÅØ£©$\frac{\underline{\;\;”÷\;\;}}{\;}$2SO2”ü+CO2”ü+2H2O£»
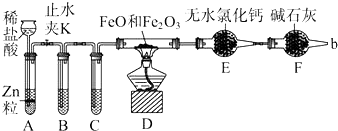
£Ø4£©¼ģŃéĘųĢåAÖŠŹĒ·ńŗ¬ÓŠH2ŗĶCO2£¬Ó¦øĆĻČ³żČ„¶žŃõ»ÆĮņ£¬ĖłŅŌ°Ń»ģŗĻĘųĢåĶعżĖįŠŌKMnO4ČÜŅŗ£¬ŌŁĶعż·ÓĢŖŹŌŅŗ¼ģŃ鶞Ńõ»ÆĮņŹĒ·ńĪüŹÕĶźČ«£¬Č»ŗó°ŃĘųĢåĶعż³ĪĒåŹÆ»ŅĖ®£¬ÓĆ³ĪĒåŹÆ»ŅĖ®¼ģŃ鶞Ńõ»ÆĢ¼£¬ŌŁĶعż¼īŹÆ»ŅøÉŌļ£¬Č»ŗóĶعż×ĘČČCuO£¬ĒāĘųÓėCuO·“Ӧɜ³ÉĖ®£¬ÓĆĪŽĖ®ĮņĖįĶ¼ģŃéĖ®µÄÉś³É£¬¼“æÉÖ¤Ć÷ÓŠĒāĘų£¬ĖłŅŌŅĒĘ÷µÄĮ¬½ÓĖ³ŠņŹĒACBEFDE£»×°ÖĆAÖŠČÜŅŗĪŖĖįŠŌKMnO4ČÜŅŗ£¬×÷ÓĆŹĒ³żČ„ĘųĢåAÖŠµÄSO2£»×°ÖĆBŹĒĒāŃõ»ÆøĘÓĆÓŚ¼ģŃ鶞Ńõ»ÆĢ¼£¬¼“ŹŌ¼ĮXµÄ»ÆѧŹ½ŹĒCa£ØOH£©2£»
¹Ź“š°øĪŖ£ŗACBEFDE£»³żČ„ĘųĢåAÖŠµÄSO2£»Ca£ØOH£©2£®
µćĘĄ ±¾Ģāæ¼²éĮĖÅØĮņĖįµÄŠŌÖŹ”¢ŹµŃé·½°øµÄÉč¼ĘÓėĘĄ¼Ū£¬ĢāÄæÄѶČÖŠµČ£¬×¢ŅāÕĘĪÕŠŌÖŹŹµŃé·½°øÉč¼ĘµÄŌŌņ¼°ĘĄ¼Ū·½·Ø£¬Ć÷Č·³£¼ūĘųĢåµÄŠŌÖŹ¼°¼ģŃé·½·Ø£¬²ąÖŲÓŚæ¼²éѧɜµÄ·ÖĪöÄÜĮ¦ŗĶŹµŃéÄÜĮ¦£®



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | Čē¹ū5.6LN2ŗ¬ÓŠnøöµŖ·Ö×Ó£¬ŌņNAŅ»¶ØŌ¼ĪŖ4n | |
| B£® | 18gĖ®ÖŠĖłŗ¬µÄµē×ÓŹżŹĒ8NA | |
| C£® | ŌŚ0.5mol/LµÄĀČ»Æ±µČÜŅŗÖŠŗ¬ÓŠĄė×ÓŹżĪŖ1.5NA | |
| D£® | 15gCH3+ŗ¬ÓŠ8molµē×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŃ”ŌńĢā
| A£® | P-P¼üµÄ¼üÄÜ“óÓŚP-Cl¼üµÄ¼üÄÜ | |
| B£® | æÉĒóCl2£Øg£©+PCl3£Øg£©ØTPCl5£Øs£©µÄ·“Ó¦ČČ”÷H | |
| C£® | Cl-Cl¼üµÄ¼üÄÜ$\frac{b-a+5.6c}{4}$ kJ•mol-1 | |
| D£® | 1molP4ŗ¬4molp-p¼ü |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
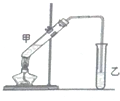 Ė×»°Ėµ£¬”°³Ā¾ĘĄĻ“×ĢŲ±šĻć”±£¬ĘäŌŅņŹĒ¾ĘŌŚ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄŅŅĖįŅŅõ„£¬ŌŚŹµŃéŹŅĄļĪŅĆĒŅ²æÉŅŌÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆĄ“Ä£ÄāøĆ¹ż³Ģ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
Ė×»°Ėµ£¬”°³Ā¾ĘĄĻ“×ĢŲ±šĻć”±£¬ĘäŌŅņŹĒ¾ĘŌŚ“¢“ę¹ż³ĢÖŠÉś³ÉĮĖÓŠĻćĪ¶µÄŅŅĖįŅŅõ„£¬ŌŚŹµŃéŹŅĄļĪŅĆĒŅ²æÉŅŌÓĆČēĶ¼ĖłŹ¾µÄ×°ÖĆĄ“Ä£ÄāøĆ¹ż³Ģ£®Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
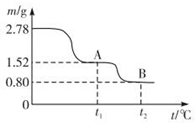
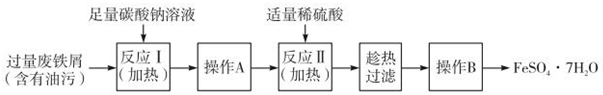
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
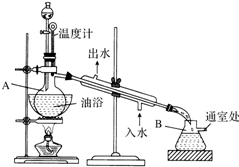 ŅŅĖįŅŅõ„ŹĒĪŽÉ«µÄÓŠĖ®¹ūĻćĪ¶µÄŅŗĢ壬·Šµć77.1”ę£¬Ä³“ĪÖĘČ”ŹµŃéÓƵ½±ł“×Ėį14.3mL£¬95%ŅŅ“¼23mL£¬»¹ÓƵ½ÅØH2SO4£¬±„ŗĶNa2CO3ČÜŅŗŅŌ¼°¼«Ņ×ÓėŅŅ“¼½įŗĻ³ÉĮł“¼ŗĻĪļµÄĀČ»ÆøĘČÜŅŗ£¬Ö÷ŅŖŅĒĘ÷×°ÖĆČēĶ¼ĖłŹ¾£¬ŹµŃé²½ÖčŹĒ£ŗ
ŅŅĖįŅŅõ„ŹĒĪŽÉ«µÄÓŠĖ®¹ūĻćĪ¶µÄŅŗĢ壬·Šµć77.1”ę£¬Ä³“ĪÖĘČ”ŹµŃéÓƵ½±ł“×Ėį14.3mL£¬95%ŅŅ“¼23mL£¬»¹ÓƵ½ÅØH2SO4£¬±„ŗĶNa2CO3ČÜŅŗŅŌ¼°¼«Ņ×ÓėŅŅ“¼½įŗĻ³ÉĮł“¼ŗĻĪļµÄĀČ»ÆøĘČÜŅŗ£¬Ö÷ŅŖŅĒĘ÷×°ÖĆČēĶ¼ĖłŹ¾£¬ŹµŃé²½ÖčŹĒ£ŗ²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ½ā“šĢā
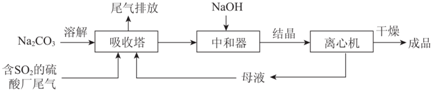
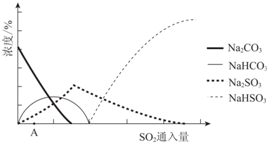
| ׏ĮĻĻŌŹ¾£ŗ ¢ń£®Na2SO3ŌŚ33”ꏱČܽā¶Č×ī“󣬽«Ę䱄ŗĶČÜŅŗ¼ÓČČÖĮ33”ęŅŌÉĻŹ±£¬ÓÉÓŚČܽā¶Č½µµĶ»įĪö³öĪŽĖ®Na2SO3£¬ĄäČ“ÖĮ33”ęŅŌĻĀŹ±Īö³öNa2SO3•7H2O£» ¢ņ£®ĪŽĖ®Na2SO3ŌŚæÕĘųÖŠ²»Ņ×±»Ńõ»Æ£¬Na2SO3•7H2OŌŚæÕĘųÖŠŅ×±»Ńõ»Æ£® |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com