
| A��Cu2O�Ǻ�ɫ���� |
| B��ұ�������е�β�������������� |
| C�����������У���2 mol CuFeS2��ȡCuʱ����������5.0mol O2 |
| D����⾫��ͭ�Ŀ�ʼ�Σ�ÿת��2mol����ʱ�������ܽ�ͭ������Ϊ64g |
 �ظ���ʦ�㲦ϵ�д�
�ظ���ʦ�㲦ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
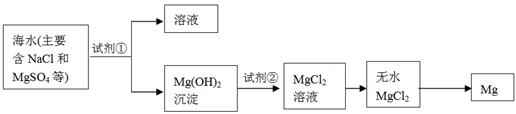

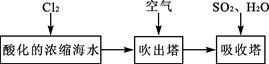
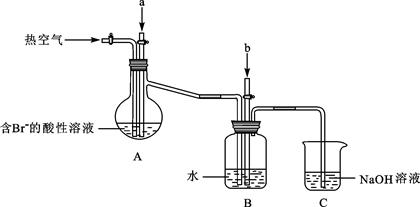
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ��ж���
 CH3OH(g) ��H1
CH3OH(g) ��H1 CH3OH(g) + H2O(g) ��H2
CH3OH(g) + H2O(g) ��H2| �¶� | 250�� | 300�� | 350�� |
| K | 2.041 | 0.270 | 0.012 |
 ��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ����
��3��ijʵ��С�����ݼ״�ȼ�յķ�Ӧԭ�����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���ٶ���ʻ��40km��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������мȿ����־��õ����ⷽ���� �������������� ����д��ţ�
���ٶ���ʻ��40km��Ϊ����Ч��չ��������Դ�����ȱ����Ƶ����۵����������мȿ����־��õ����ⷽ���� �������������� ����д��ţ� ��4��������������ȼ������ˮ���Ȼ�ѧ����ʽ
��4��������������ȼ������ˮ���Ȼ�ѧ����ʽ 2H2��g��+ O2(g) ="=" 2H2O(g) ��H��a kJ/mol
2H2��g��+ O2(g) ="=" 2H2O(g) ��H��a kJ/mol 2H2��g��+ O2(g) ==2H2O(l) ��H��b kJ/mol
2H2��g��+ O2(g) ==2H2O(l) ��H��b kJ/mol
 ����2 molH2��ȫȼ������ˮ��������ų����������������������������������b��kJ
����2 molH2��ȫȼ������ˮ��������ų����������������������������������b��kJ ����֪��1mol�����еĻ�ѧ��Ҫ����436 kJ��������1mol�����еĻ�ѧ��Ҫ
����֪��1mol�����еĻ�ѧ��Ҫ����436 kJ��������1mol�����еĻ�ѧ��Ҫ ����496kJ������ˮ������1molH��O���γ�ʱ�ų�����463kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��
����496kJ������ˮ������1molH��O���γ�ʱ�ų�����463kJ��д���÷�Ӧ���Ȼ�ѧ����ʽ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����ֲ��������ȡ�� |
| B���ú�ˮ��ȡ��ʳ�κ͵�ˮ |
| C���ÿ�ȼ����ȡ��������ȼ�� |
| D���û�ͭ����Ҫ�ɷ�ΪCu2S��ұ������ͭ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A����Ϊ����ԭ�ϣ���Ȼ����Ҫ���ںϳɰ��������״��� |
| B�����ݹ����������Һ�Ĺ����ܱ��ʹ�ʵ�ͻ��� |
| C�����������������������ʹ������Ȼ�̼��Һ��ɫ |
| D�����ۺ���ά����ȫˮ��IJ�����ͬ |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com