
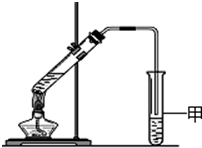
 ĶعżĮøŹ³·¢½ĶæÉ»ńµĆijŗ¬ŃõÓŠ»śĪļX£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ46£¬ĘäÖŠĢ¼µÄÖŹĮæ·ÖŹżĪŖ52.2%£¬ĒāµÄÖŹĮæ·ÖŹżĪŖ13.0%£®
ĶعżĮøŹ³·¢½ĶæÉ»ńµĆijŗ¬ŃõÓŠ»śĪļX£¬ĘäĻą¶Ō·Ö×ÓÖŹĮæĪŖ46£¬ĘäÖŠĢ¼µÄÖŹĮæ·ÖŹżĪŖ52.2%£¬ĒāµÄÖŹĮæ·ÖŹżĪŖ13.0%£®| 46”Į52.2% |
| 12 |
| 46”Į13.0% |
| 1 |
| 46”Į34.8% |
| 16 |
| Cu |
| ”÷ |
| Cu |
| ”÷ |


 ÓäæģµÄŗ®¼ŁÄĻ¾©³ö°ęÉēĻµĮŠ“š°ø
ÓäæģµÄŗ®¼ŁÄĻ¾©³ö°ęÉēĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ

²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
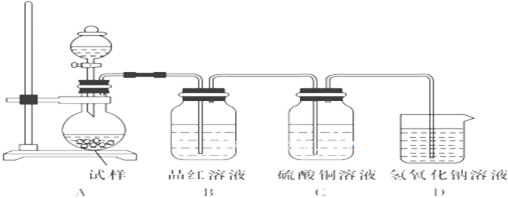
 ĖÄÖÖ¶ĢÖÜĘŚŌŖĖŲA”¢B”¢C”¢DŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĶ¼ĖłŹ¾£¬ĘäÖŠDŠĪ³ÉµÄĮ½ÖÖŃõ»ÆĪļ¶¼ŹĒ“óĘųĪŪČ¾Īļ£®ĻĀĮŠÓŠ¹ŲÅŠ¶ĻÕżČ·µÄŹĒ£Ø””””£©
ĖÄÖÖ¶ĢÖÜĘŚŌŖĖŲA”¢B”¢C”¢DŌŚŌŖĖŲÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĶ¼ĖłŹ¾£¬ĘäÖŠDŠĪ³ÉµÄĮ½ÖÖŃõ»ÆĪļ¶¼ŹĒ“óĘųĪŪČ¾Īļ£®ĻĀĮŠÓŠ¹ŲÅŠ¶ĻÕżČ·µÄŹĒ£Ø””””£©| A”¢¼ņµ„Ēā»ÆĪļµÄČČĪČ¶ØŠŌ£ŗC£¾A |
| B”¢×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄĖįŠŌ£ŗD£¼C |
| C”¢BµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļÄÜÓėDµÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļ·“Ó¦ |
| D”¢A”¢C”¢DµÄ×īøß¼Ūŗ¬ŃõĖįµÄÄĘŃĪĖ®ČÜŅŗæÉÄܾłĻŌ¼īŠŌ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
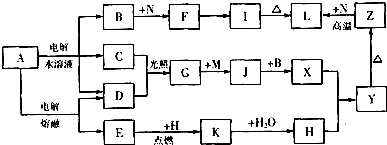
| ŌŖĖŲ | Ļą¹ŲŠÅĻ¢ |
| X | XµÄŅ»ÖÖŗĖĖŲµÄÖŹĮæŹżĪŖ18£¬ÖŠ×ÓŹżĪŖ10 |
| Y | YÓėXĶ¬Ö÷×壬ĒŅĖüµÄŅ»ÖÖŃõ»ÆĪļŹĒµ¼ÖĀĖįÓźµÄÖ÷ŅŖĪļÖŹÖ®Ņ» |
| Z | ZµÄµ„ÖŹ³£ĪĀĻĀĪŖ»ĘĀĢÉ«ĘųĢå |
| W | WµÄ»łĢ¬Ō×ÓŗĖĶāÓŠ4øöĪ“³É¶Ōµē×Ó |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
| A”¢²»ŗ¬NO3-£¬Ņ²²»ŗ¬Fe3+ |
| B”¢ŗ¬ÓŠNO3-”¢I-”¢Cl? |
| C”¢ŗ¬I-£¬ĒŅæĻ¶Øŗ¬ÓŠCl- |
| D”¢æÉÄÜŗ¬ÓŠFe3+ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
A”¢ŌŚÕōĮóÉÕĘæÖŠŹ¢Ō¼
| ||
| B”¢ŹÕ¼ÆÕōĮóĖ®Ź±£¬Ó¦ĘśČ„æŖŹ¼Įó³öµÄ²æ·Ö | ||
| C”¢ĄäĖ®“ÓĄäÄż¹ÜĻĀæŚČė£¬ÉĻæŚ³ö | ||
| D”¢½«ĪĀ¶Č¼ĘĖ®ŅųĒņ²åČė×ŌĄ“Ė®ÖŠ |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com