
���� �������Ũ�ȵĴ�����NaOH��Һ����ǡ�÷�Ӧ���ɴ�������Һ����Һ�д��������ˮ���Լ��ԣ�c��OH-����c��H+����
���-�����Ĵ�����������ƻ�Ϻ���Һ��pH=7�������Һ�е���غ������c��OH-��=c��H+����
��� �⣺�������Ũ�ȵĴ�����NaOH��Һ����ǡ�÷�Ӧ���ɴ�������Һ����Һ�д��������ˮ���Լ��ԣ�c��Na+����c��CH3COO-����c��OH-����c��H+����
���-�����Ĵ�����������ƻ�Ϻ���Һ��pH=7��c��OH-��=c��H+���������Һ�е���غ������c��CH3COO-��+c��OH-��=c��Na+��+c��H+����c��Na+��=c��CH3COO-�����õ���Һ������Ũ�ȴ�СΪ��c��Na+��=c��CH3COO-����c��OH-��=c��H+����
�ʴ�Ϊ��A��c��Na+��=c��CH3COO-����c��OH-��=c��H+����
���� ���⿼���˵������Һ������Ũ�ȴ�С�ȽϷ�����ע������ˮ������͵���غ��Ӧ�ã����ջ����ǹؼ�����Ŀ�ϼ�


 �Ķ��쳵ϵ�д�
�Ķ��쳵ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��������������Ϊ5 | B�� | ���Ľ����Ա�Ǧǿ | ||
| C�� | ������+2��+3��+4�� | D�� | ��������������ˮ������ǿ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 1.0L1.0mo1•L-1��NaAlO2ˮ��Һ�к��е���ԭ����Ϊ2NA | |
| B�� | 1 mol������1 moL NO2��������������ͬ | |
| C�� | 1L pH=13��NaOH��Һ�к���OH-����Ŀһ��Ϊ0.1 NA | |
| D�� | 12g���ʯ�к���C-C����ĿΪ2NA |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
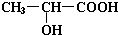 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | 21��5 | B�� | 4��1 | C�� | 3��1 | D�� | 11��3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
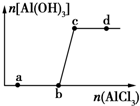 ��һ������NaOH��Һ����μ���AlCl3��Һ�����ɳ���Al��OH��3������AlCl3�������ı仯��ϵ��ͼ��ʾ���������������ڶ�Ӧ����Һ��һ���ܴ���������ǣ�������
��һ������NaOH��Һ����μ���AlCl3��Һ�����ɳ���Al��OH��3������AlCl3�������ı仯��ϵ��ͼ��ʾ���������������ڶ�Ӧ����Һ��һ���ܴ���������ǣ�������| A�� | a���Ӧ����Һ�У�Na+��Mg2+��SO42-��HCO3- | |
| B�� | b���Ӧ����Һ�У�Ag+��Ca2+��NO3-��F- | |
| C�� | c���Ӧ����Һ�У�Na+��S2-��SO42-��Cl- | |
| D�� | d���Ӧ����Һ�У�K+��NH4+��I-��CO32- |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com