
��NA��ʾ�����ӵ���������ֵ������������ȷ����
��0.84 g ��������������Ϊ0.01NA
��������������Ϊ0.01NA
�ڳ����£�1L0��1 mol��L-1 Na2CO3��Һ����������Ŀ֮�ʹ���0��1 NA
�۳��³�ѹ�£�0��3molCl2ͨ��ˮ�У���ַ�Ӧ��ת�Ƶ�����ĿΪ0��3 NA
��7.8g Na2S��Na2O2�Ļ�����к��е�������������0.1 NA
�ݱ�״���£�22��4LSO3 �ķ�����ΪNA
�ޱ�״���£�22��4LNO��11��2L O2��Ϻ�����ķ�����������NA
A���٢ڢ� B���٢ڢ� C���ڢܢ� D���ۢܢ�
B
��������
�����������0.84 g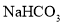 ��������ʵ�����0.01mol�����е���������̼��������ӣ���ĿΪ0.01NA
��������ʵ�����0.01mol�����е���������̼��������ӣ���ĿΪ0.01NA
����ȷ����Na2CO3��Һ��̼�������ˮ��ʹ��������Ŀ���࣬����1L0��1 mol��L-1 Na2CO3��Һ����������Ŀ֮�ʹ���0��1 NA����ȷ����������ˮ�ķ�Ӧ�ǿ��淴Ӧ��0.3mol��������ȫ���μӷ�Ӧ������Na2S��Na2O2��Ħ��������ͬ�����Ի�����ƽ��Ħ����������78g/mol������7.8g�û��������ʵ�����0.1mol�����������ӵ���Ŀ��0.1NA����ȷ���ݱ�״�����������������壬����22��4LSO3 �ķ���������NA������NO��O2��Ӧǡ�����ɶ�����������NO2��N2O4֮�����ƽ��ת��������ʹ���������С��NA������ѡB��
���㣺���鰢��٤��������Ӧ��


 ѧ���쳵�����ּ��ں�����ҵϵ�д�
ѧ���쳵�����ּ��ں�����ҵϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ������ѧ�ڵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
�����о�Ŀ�ĺ�ʾ��ͼ�������( )
| A | B | C | D |
�� �� Ŀ �� | �̶��ݻ����ܱ������У�ѹǿ�Է�Ӧ�� 2SO2(g)+O2(g) | �̶��ݻ����ܱ������У��¶ȶԷ�Ӧ�� N2(g) +3H2(g) | �̶��ݻ����ܱ������У�����CO2Ũ�ȣ����Ѵ�ƽ��ķ�Ӧ�� CO(g)+H2O(g) | ������Na��K �ֱ�������ˮ��Ӧ |
ʾ �� ͼ |
|
|
|
|
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�ϲ�����У�����ϵ�һ��������ѧ�Ծ��������棩 ���ͣ�ѡ����
���±���ʾ��Ϊ�ᴿ�������ʣ�������Ϊ�������ʣ�����ѡ�õij����Լ�����Ҫ���뷽������ȷ����
| �������� | �����Լ� | ���뷽�� |
A | �����ױ��� | KMnO4���ữ����NaOH��Һ | ��Һ |
B | NH4Cl��Һ��FeCl3�� | NaOH��Һ | ���� |
C | �������������ᣩ | KOH��Һ��ˮ | ��Һ |
D | CO3��SO2�� | Na2CO3 | ϴ�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ������ѧ�ڵ�һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ������
(12��)������������������(��Ҫ�ɷ�ΪFe2O3��Fe3O4��FeO��SiO2)Ϊԭ���Ʊ��ߴ�����������������ʾ��ͼ��
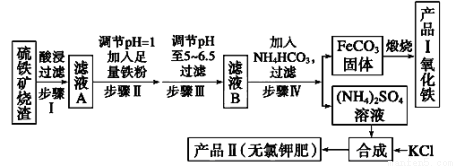

�ش��������⣺
��1����������˺���ҺA�еĽ�����������_______________________ _______��
��2����ҺB�м���NH4HCO3��Һ�����ӷ���ʽ ��
��3������FeCO3���ɲ�ƷI�Ļ�ѧ��Ӧ����ʽΪ___________ __________________��
��4����֪�����ε��ܽ�����¶ȱ仯����������ͼ��ʾ����Ʒ��Ļ�ѧʽΪ��������������Ϊ�˻�ò�Ʒ����(NH4)2SO4��Һ�м���KCl��Һ����Ҫ���еIJ����ǡ������������������ȹ��ˡ�ϴ�ӡ����
��5�������Ʒ�������Ƿ����������Ȼ����������õ����Լ���_______________����һ���ᴿ��Ʒ��ķ�����________________��
��6��������п�ѡ��______________(�����)�Լ�������Һ��pH��
A��ϡ���� B��˫��ˮ C����ˮ D�����������Һ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ������ѧ�ڵ�һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
�ڹ�����ϡ������Һ�м���5.6 g Fe�ۣ�����Ӧ��ȫ���ټ���50 mL0.5mol��L-1 KNO3��Һ��ǡ�÷�Ӧ��ȫ���÷�Ӧ�ķ���ʽΪ��
�� FeSO4 + KNO3 + H2SO4�� K2SO4 + Fe2(SO4)3+ NxOy + H2O������Ը÷�Ӧ������˵����ȷ����
A����Ӧ�л�ԭ������NO
B����������ͻ�ԭ��������ʵ���֮��Ϊ1��4
C����Ӧ������ת�Ƶĵ�����Ϊ8e-
D����ѧ����ʽ�а�����˳��ļ������ǣ�8��2��5��1��4��1��5
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ������ѧ�ڵ�һ�ο��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ������(������������)�������Ӧʵ���һ����
ѡ�� | ʵ������(ʡ�Լг�װ��) | ��Ӧʵ�� l |
A | ���żܡ������ǡ�����������ǯ | ��CuSO4 |
B | �ձ�������������ͷ�ιܡ�����ƿ | ��Ũ��������0.1mol��L-1��HCl��Һ |
C | �ձ�������������Һ©�� | �ñ���Na2CO3��Һ��ȥ���������е�������Ҵ� |
D | �ձ�����ʽ�ζ��ܡ���ʽ�ζ��� | ��H2SO4��Һ�ζ�δ֪Ũ�ȵ�NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ������ѧ�ڵ�һѧ���¿���ѧ�Ծ��������棩 ���ͣ������
��12�֣���1������A��B��C���ֻ������ȡ40g���ϣ���ȫ��Ӧ��18g B��49g C ������D���ɡ���֪D��ʽ��Ϊ106���ֽ�22g A��11g B��Ϸ�Ӧ��������D mol��
��2��200mLij��Һ�к��е����Ӽ������ʵ������£�
���� | H+ | K+ | NO3- | SO42- |
���ʵ�����mol�� | 0.5 | 0.1 | 0.6 | 0.15 |
����Һ�л����е�����һ���������е� ������ţ��������ӵ�Ũ��Ϊ
A��Al3+ B��Cl- C ClO- D�� Fe2+
��3��ijѧ����һ֧�Թ��а�һ����˳��ֱ�������м�������(һ������ֻ��һ��)��
A��KI��Һ B��������Һ C��NaOH��Һ D��ϡH2SO4 E����ˮ
������Һ��ɫ������˳��仯������ɫ�����ػ�ɫ������ɫ������ɫ������ɫ��������Һ��ɫ�ı仯���ش��������⣺
(��)��������ҩƷ��˳����(д���) ____________________________________________��
(��)�١��ڷ�Ӧ�Ļ�ѧ����ʽΪ_______________________________________________��
(��)��Һ���ػ�ɫ��Ϊ��ɫ��ԭ����___________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭����У������һ��������ѧ�Ծ��������棩 ���ͣ�ʵ����
��13�֣��Ҷ���ͨ���Զ�ˮ�������ʽ���ڣ��׳Ʋ��ᾧ�壮��֪���ᾧ����101��ʱ�ۻ�����ʼ������157��ʱ�����������������»�ֽ�����CO��CO2��H2O��
��1�����й����Ҷ����������ȷ���ǣ����ţ�________________��
���ܺ��Ҷ�������������Ӧ ����ʹ���Ը��������Һ��ɫ
������Һ��ʹ��ɫʯ����ֽ��� ���ܺ�̼��������Һ��Ӧ��������
��2���Ҷ���Ĺ�ҵ��������֮һ�����Ҷ���Ϊԭ�ϣ���һ�������£��ÿ��������õ���д���÷�Ӧ�Ļ�ѧ��Ӧ����ʽ ��_____________________________________________________________________��
��3����������ᾧ�����ȷֽ�IJ������Ƿ���CO2���ס�����λͬѧ�ֱ������װ��1��װ��2�����ʵ�飮

��I����������װ��1�����ԵIJ������� ��_________________________________________________��
��II��B��E����װ���и���������________������Ϊ�� __________________________________ ��
��III�����A��F��ѡ����ʵ���������װһ�ɸ��ôﵽʵ��Ŀ��װ�ã��������������ҵ�˳������Ϊ������ĸ��ʾ����_________________��
������һ�������²�����立ֽ�����NH3��CO��CO2��H2O��110��ʱ���û��������ͨ��Na2O2����ͨ��Ũ����ֱ�����Ag��Bg �� �������������ͨ��Ũ���ᣬ��ͨ��Na2O2�ֱ�����Cg��Dg���ܷ�Ӧ���������������ȫ���Ҹ�������CO��Na2O2����Ӧ������A��B��C��D�Ĵ�С��ϵΪ��
___________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�콭��ʡ�����и���9��ѧ����л�ѧ�Ծ��������棩 ���ͣ�ѡ����
��ѧ�뻷�������ϡ���Ϣ����Դ��ϵ���У�����˵����ȷ����
A���뵼����ҵ����һ�仰������ɳ̲���û����������оƬ�IJ����Ƕ�������
B����ɫ��ѧ�ĺ��������û�ѧԭ������������Ⱦ
C����ú������Һ����������ȼ��
D��PM2.5��ָ������ֱ���ӽ���2.5��10��6 m�Ŀ����Ҳ��ϸ�������Щϸ�������ɢ�ڿ������γɻ������ж����ЧӦ
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com