
��������ϸ����һ�ֺ��Ǻ˵���������Ҫ��rRNA�͵����ʹ��ɣ���Ψһ�����ǰ���mRNA��ָ�������ϳɵ����ʶ����������Ժ�������ϸ���ڵ����ʺϳɵķ��ӻ���������˵������ȷ���� (����)��
A������͵����ʶ���������Ҫ��Ӫ������
B����������һ���������ܷ���ˮ�ⷴӦ�����ɰ�����
C��������ɱ������H1N1���в�������Ϊ�����ĵ��������ȱ���
D����������Һ�м��뱥���������Һ�����������������ټ�ˮҲ���ܽ�
 ���㼤�������100�ִ��Ծ�ϵ�д�
���㼤�������100�ִ��Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
д�����е������ʵĵ��뷽��ʽ
(1)H2SO4______________________________________________________________________��
(2)H2CO3______________________________________________________________________��
(3)Ca(OH)2____________________________________________________________________��
(4)Fe(OH)3_____________________________________________________________________��
(5)NH3·H2O____________________________________________________________________��
(6)NaCl_______________________________________________________________________��
(7)BaSO4_____________________________________________________________________��
(8)NaHSO4_____________________________________________________________________��
(9)NaHCO3____________________________________________________________________��
(10)NaHSO4(����)_____________________________________________________________��
(11)Al2O3(����)___________________________________________________________��
(12)CH3COOH_______________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���б�ʾ��Ӧ��ѧ��Ӧ�����ӷ���ʽ��ȷ����(����)
A����KIO3����������Һ�е�KI��5I����IO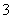 ��3H2O===3I2��6OH��
��3H2O===3I2��6OH��
B����NH4HSO3��Һ�мӹ�����NaOH��Һ�����ȣ�NH ��OH��
��OH��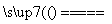 NH3����H2O
NH3����H2O
C��������SO2ͨ���䰱ˮ�У�SO2��NH3·H2O===HSO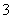 ��NH
��NH
D��Ba(OH)2��Һ����μ���һ�����ʵ���Ũ�ȵ�NaHSO4��Һ������Һ�е�OH��ǡ�÷�Ӧһ��ʱ��Ba2����2OH����2H����SO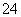 ===BaSO4����2H2O
===BaSO4����2H2O
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�������ӷ���ʽ��ȷ����(����)
A��Fe�����ᷴӦ��H2��2Fe��6H��===2Fe3����3H2��
B����ʯī�缫��ⱥ��ʳ��ˮ��2H����2Cl��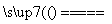 Cl2����H2��
Cl2����H2��
C����AlCl3�Ͱ�ˮ�Ʊ�Al(OH)3��Al3����3OH��===Al(OH)3��
D����Mg(OH)2��Һ�е���FeCl3��Һ��3Mg(OH)2��2Fe3��2Fe(OH)3��3Mg2��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�߾���Ľṹ��ͼ��ʾ������˵����ȷ����(���� )
)

A���ø߾���Ϊ�Ӿ۲���
B���ø߷���Ϊ���߷���
C���ø߷��ӵĵ�����6��
D���ø߷����к����������ǻ����Ȼ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���й��ھ����˵����һ����ȷ���� (����)��
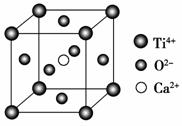
CaTiO3�ľ���ṹģ��(ͼ��Ca2����O2����
Ti4���ֱ�λ������������ġ����ĺͶ���)
A�����Ӿ����ж����ڹ��ۼ�
B������ͼ��CaTiO3������ÿ��Ti4����12��O2�������
C��SiO2������ÿ����ԭ����������ԭ���Թ��ۼ�����
D������������۵㶼�ȷ��Ӿ�����۵��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
Ԫ�ص�ԭ�Ӱ뾶�����ڱ��г��������Ա仯�ĸ���ԭ����(����)
A��ԭ�ӵ����ԭ���������������Ա仯
B��Ԫ�صĻ��ϼ۳��������Ա仯
C��ԭ�ӵ����ʳ��������Ա仯
D��Ԫ��ԭ�ӵĺ�������Ų��������Ա仯
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com