
��ҵ����������Ϊԭ���Ʊ��������ѵĹ�����������ͼ��ʾ�����������Ҫ�ɷ�Ϊ��������(FeTiO3)������һ������Ԫ���ڷ绯�����л�ת��Ϊ��3�ۡ�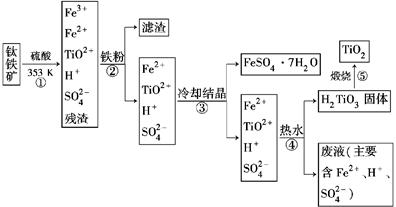
��֪��TiOSO4��ˮ��ˮ�⡣
(1)������У������۽�Fe3��ת��ΪFe2�������ӷ���ʽΪ_______________________
(2)������У�ʵ�ֻ����ķ������������ʵ�________(����ĸ���)��
a���ۡ��е���졡 b���ܽ��Բ��졡 c�������ԡ���ԭ�Բ���
(3)����ڡ��ۡ����У�����Ҫ���еIJ�����________(���������)��
(4)���ϻ�ѧ�����û�ѧƽ�����۽��Ͳ�����н�TiO2��ת��ΪH2TiO3��ԭ����
____________________________________________________________��
(5)�������������еķ�Һ�����̿�(��Ҫ�ɷ�ΪMnO2)��Ӧ������������(MnSO4��������ˮ)���÷�Ӧ�����ӷ���ʽΪ__________________________________
(6)�о����֣���ʯī������������������������CaF2��CaO������ʣ�������ͼ��ʾװ�ÿɻ�ý����ƣ������Ը�Ϊ��ԭ������ԭ���������Ʊ������ѡ�
д�������ĵ缫��Ӧʽ��_________________________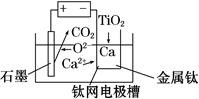
���Ʊ�������ǰ��CaO���������䣬��ԭ����______________________________________(���ϻ�ѧ�������)��
(1)2Fe3����Fe===3Fe2����(2)b��(3)����
(4)��Һ�д���ƽ�⣺TiO2����2H2O??H2TiO3��2H������������ˮϡ�͡����º�ƽ�������ƶ�������H2TiO3��(5)MnO2��2Fe2����4H��=Mn2����2Fe3����2H2O��(6)��2O2����4e��=O2��(��C��2O2����4e��=CO2��)�����Ʊ�Tiʱ��������Ӧ��2CaO 2Ca��O2����2Ca��TiO2
2Ca��O2����2Ca��TiO2 Ti��2CaO���ɴ˿ɼ���CaO����������(���Ʊ�Tiʱ������������Ӧ��2Ca2����4e��===2Ca������������Ӧ��2O2����4e��===O2����2Ca��TiO2
Ti��2CaO���ɴ˿ɼ���CaO����������(���Ʊ�Tiʱ������������Ӧ��2Ca2����4e��===2Ca������������Ӧ��2O2����4e��===O2����2Ca��TiO2 Ti��2CaO���ɴ˿ɼ���CaO����������)
Ti��2CaO���ɴ˿ɼ���CaO����������)
����



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
ȡA��B�������ʵ���Ũ����ȵ�NaOH��Һ�������Ϊ50 mL���ֱ�������ͨ��һ������CO2���ٷֱ�ϡ��Ϊ100 mL��
(1)��NaOH��Һ��ͨ��һ������CO2����Һ�е����ʵ���ɿ����ǣ�
�� ���� ���� ���� ��
(2)��ϡ�ͺ����Һ�зֱ���μ���0.1 mol��L��1�����ᣬ������CO2�����(��״��)����������������ϵ��ͼ��ʾ��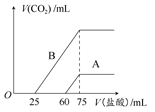
�ٷֱ�����������������Һ�е������� ��ԭNaOH��Һ�����ʵ���Ũ���� ��
��A���߱�����ͨ��CO2����Һ�е������� �������ᷴӦ����CO2���������� mL(��״��)��
��B���߱�����ԭNaOH��Һͨ��CO2���������ʵĻ�ѧʽΪ �������ʵ���֮��Ϊ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������������һ����Ҫ�Ļ��������м����,��Ҫ�ɷ���Fe3O4��Fe2O3��FeO�Ͷ�������ȡ��������������������Ʊ���Ч��ˮ���ۺ�������������ͼ: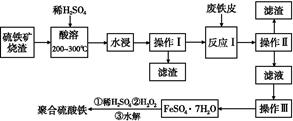
(1)ʵ����ʵ�֡����������õIJ����������������������������ձ�����������ϵ�в�����������Ϊ�����������������������˺�ϴ�ӡ�
(2)�����ܡ�������Fe3O4�ܽ�Ļ�ѧ��Ӧ����ʽΪ ��
(3)�����������ڡ����ܡ�ǰҪ�������ҪĿ���� ��
(4)ʵ���Ҽ��顰��Ӧ���Ѿ���ȫ���Լ�����������,������ ��
(5)��������H2O2��Ŀ��������Fe2+,д��H2O2����Fe2+ΪFe3+�����ӷ���ʽ: ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�ҹ���ѧ�Һ�°�ĸ����Ĵ�����������,�������̿ɼ�Ҫ��ʾ��ͼ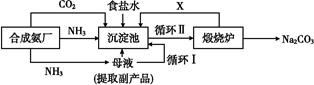
(1)������������ķ�������������,����Ʒ��һ����;Ϊ����������
(2)�������з����Ļ�ѧ��Ӧ����ʽ�� ����
(3)д������������X���ʵķ���ʽ�� ��
(4)ʹԭ���Ȼ��Ƶ������ʴ�70%��ߵ�90%����,��Ҫ���������������(�����������еı��)��ѭ�����ӳ�������ȡ�������IJ������������� ��
(5)Ϊ�����Ʒ̼�������Ƿ����Ȼ���,��ȡ������������ˮ��,�ٵμ�����������
(6)��ĸҺ��ͨ����,����ϸСʳ�ο���,��ȴ��������Ʒ,ͨ����������������������
a.����NH4+��Ũ��,ʹNH4Cl���������
b.ʹNaHCO3���������
c.ʹNaHCO3ת��ΪNa2CO3,���������NH4Cl����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
���ӹ�ҵ����30%��FeCl3��Һ��ʴ���ھ�Ե���ϵ�ͭ��,����ӡˢ��·�塣ij����ʦΪ�˴ӷ�Һ�л���ͭ,���»��FeCl3��Һ,���������ʵ�鲽��: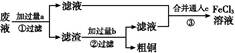
д��һ����֤����ԭ��Fe��Cuǿ�����ӷ���ʽ: ��
�÷�Ӧ����ͼ���� �з����������������Ӧ���һ��ԭ���,�ڷ����л�������װ��ͼ(����缫���ơ��缫���ϡ��������Һ)��
| |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
����ת����ϵ�У�X��Y����������;�㷺�����ֽ������ʣ�A��B�������A�ʺ���ɫ��C��D��E����ѧ���������ֻ��������ת����ϵ�ش����⣺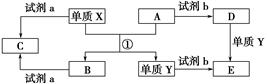
��1����д����Ӧ�ٵĻ�ѧ����ʽ___________________________________________��
��2������D��Һ��Y���ӵķ�����_____________________________________��
��3�����Լ�a��NaOH��Һ��д������X��NaOH��Һ��Ӧ�����ӷ���ʽ______________________________��
��4�����Լ�b��H2SO4����ҵ����E��H2SO4��NaNO2Ϊԭ����ȡ��Ч��ˮ��Y��OH��SO4����֪��ԭ����ΪNO����÷�Ӧ�Ļ�ѧ����ʽ��_____________________________________________________________________��
��5����ҵ�ϵ�����ڵ�B��ȡXʱ�������������������ڱ�״���µ����Ϊ33.6 m3�����������������Ϊ________kg��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
������ȡ��������Ŀ�������Ҫ�ɷ�ΪFe2O3��Fe3O4��FeO��Al2O3��SiO2�ȣ��Ʊ��ߴ�����������-Fe2O3���Ĺ����������£�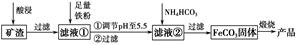
��1���������������Fe3O4������Ӧ�����ӷ���ʽΪ______________________________��Ϊ��ߡ����������Ԫ�صĽ����ʣ����˲��ú��ʵ�Һ�̱Ⱥ�ѭ����ȡ�⣬���˵���������____________________��____________________�����ξ�������
��2������pH��5.5��Ŀ����______________________________________��
��3����Һ���м���NH4HCO3ʱ��Ҫ���Ʒ�Ӧ�¶Ȳ��ܹ��ߣ�ԭ����__________________________________________________������һ�㼴�ɣ�
��4���ڿ���������FeCO3�Ʊ��ߴ��������Ļ�ѧ����ʽΪ_______________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��ȸʯ��Ҫ��Cu2(OH)2CO3�������������Ļ������Ļ�����Կ�ȸʯΪԭ�Ͽ��Ʊ�CuCl2��3H2O�����������ͼ��ʾ��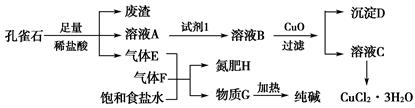
��֪����ҺAֻ��Cu2����Fe2����Fe3�����ֽ������ӣ����������ӳ���ʱ��pH�����ʾ���ش��������⣺
| �������� | Fe3�� | Fe2�� | Cu2�� | |
| pH | �������↑ʼ���� | 1.9 | 7.0 | 4.7 |
| ����������ȫ���� | 3.2 | 9.0 | 6.7 | |
 Cu(OH)2��2H����ƽ�ⳣ��Ϊ________��
Cu(OH)2��2H����ƽ�ⳣ��Ϊ________���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
��2.0 mol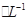 CuSO4��1.0 mol
CuSO4��1.0 mol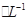 H2SO4��Һ�������ϣ������Ϻ����Һ��������ڻ��ǰ������Һ�����֮�ͣ����㣺
H2SO4��Һ�������ϣ������Ϻ����Һ��������ڻ��ǰ������Һ�����֮�ͣ����㣺
��1�������Һ��CuSO4��H2SO4�����ʵ���Ũ��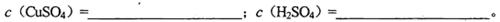
��2�����Һ�к͵����ʵ���Ũ��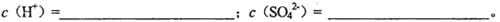
��3������Һ�м������ۣ������㹻����ʱ�䣬������ʣ�ࡣ��ʱ��Һ�е����ʵ���Ũ�ȡ�c(Fe2��)=_______________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com