
������ƿ��ʧ��ǩ��NaOH��Na2CO3��AlCl3��NH4HSO4��Һ��Ϊ������ƿ��Һ������ƿ��Һ���ΪA��B��C��D����ʵ�飮ʵ����̺ͼ�¼����ͼ��ʾ(�������Ѿ���ȥ)��

��ش�
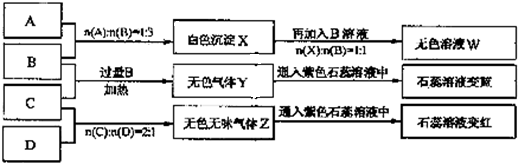
(1)X��B��Ӧ�����ӷ���ʽΪ________��
(2)D��ҺpH________(����ڡ�����С�ڡ����ڡ�)7��ԭ����(�����ӷ���ʽ��ʾ)________��
(3)�����ʵ���Ũ�ȵ�A��B��C��D��ҺpH�ɴ�С��˳����________��(�û�ѧʽ��ʾ)
(4)��д��C�����B��Ӧ(����)�����ӷ���ʽ________��
(5)��B��C��ϡ��Һ��Ϻ�(������)��Һ�����ԣ������Һ������Ũ�ȴӴ�С��˳����________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ||
| ||
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

��������и��⣺
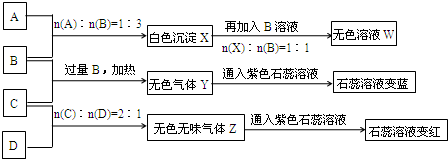
(1)Y��Z�Ļ�ѧʽ�ֱ�ΪY___________��Z___________��X��B��Ӧ�����ӷ���ʽΪ____________________��
(2)D��ҺpH______________7(����ڡ���С�ڡ����ڡ�)��ԭ����(�����ӷ���ʽ��ʾ)____________________________________________________________________��
(3)�����ʵ���Ũ�ȵ�A��B��C��D��ҺpH�ɴ�С��˳����____________________��(�û�ѧʽ��ʾ)
(4)��д��C�����B��Ӧ(����)�����ӷ���ʽ____________________��
(5)��B��C��ϡ��Һ��Ϻ�(������)��Һ�����ԣ������Һ������Ũ�ȴӴ�С��˳����____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com