
���� ��1������ҪCuSO4•5H2O������Ϊx��H2O������Ϊy�����ݻ�ѧʽ���������ͭ������m��CuSO4������Һ����Ϊx+y���������������з��̣�����x��y�ı�����ϵ��
��2�����ݹ�ϵʽ����n��CuCl��������������Ʒ��m��CuCl��������0.2500g�ϸ��CuCl�к���CuCl�����������бȽ��жϣ�
��� �⣺��1������ҪCuSO4•5H2O������Ϊx��H2O������Ϊy����$\frac{\frac{160}{250}x}{x+y}$��100%=20.0%����16x=5��x+y�������� x��y=5��11��
������CuSO4•5H2O��H2O������֮��Ϊ5��11��
��2������Ʒ��CuCl������Ϊx����
�ɻ�ѧ��Ӧ����ʽ��֪��CuCl������Fe2+������Ce4+
1 1
n��CuCl�� 24.60��10-3L��0.1000 mol/L
���� n��CuCl��=24.60��10-3L��0.1000 mol/L=2.46��10-3mol�����Ը���ƷCuCl������Ϊ2.46��10-3mol��99.5g/mol=0.2448g��
0.2500g�ϸ��CuCl�к���CuCl������0.2500g��96.5%=0.2413g��С��0.2448g���ʸ���Ʒ��CuCl�������������ϱ���
����Ʒ��CuCl�������������ϱ���
���� ���⿼�����ʵ���Ũ�ȼ��㡢�ζ�����ȣ��Ѷ��еȣ�ע���ڵζ������������漰��Ӧ�϶࣬ͨ�����ù�ϵʽ���㣮



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
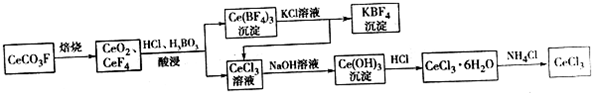
 ����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128�桫135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ�����£�
����������Ӧ����㷺�Ľ��ȡ���ʹ�Ϳ���ҩ������ˮ���������ֽ⣬�ֽ��¶�Ϊ128�桫135�森ijѧϰС����ʵ������ˮ���ᣨ���ǻ������ᣩ�������Ϊ��Ҫԭ�Ϻϳɰ�˾ƥ�֣���Ӧԭ�����£�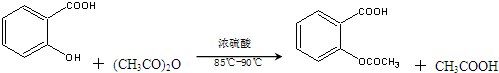
| ���� | ��Է������� | �۵��е㣨�棩 | ˮ���� |
| ˮ���� | 138 | 158���۵㣩 | �� |
| ������ | 102 | 139.4���е㣩 | ��ˮ�� |
| ����ˮ���� | 180 | 135���۵㣩 | �� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ʵ����
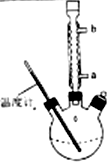
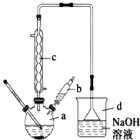 �屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�
�屽��һ�ֳ��õĻ���ԭ�ϣ�ʵ�����Ʊ��屽��ʵ�鲽�����£�| �� | �� | �屽 | |
| �ܶ�/gcm-3 | 0.88 | 3.10 | 1.50 |
| �е�/�� | 80 | 59 | 156 |
| ��ˮ�е��ܽ�� | �� | �� | �� |
 ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ij��ѧѧϰС����о������ǣ�̽���ⶨ���ᾧ�壨H2C2O4•xH2O����x��ֵ������ͬѧͨ���������ϲ�Ѱ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���
ij��ѧѧϰС����о������ǣ�̽���ⶨ���ᾧ�壨H2C2O4•xH2O����x��ֵ������ͬѧͨ���������ϲ�Ѱ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ����鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | ��ʽ�ζ���δ�ô�����Һ��ϴ | |
| B�� | ��ʽ�ζ���δ�ô�װ��Һ��ϴ | |
| C�� | ��ƿδ�ô�װ��Һ��ϴ | |
| D�� | �ڵζ�ǰ�ζ��ܼ��첿�������ݣ��ζ���������ʧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ʱ�� | c��CO��/mol•L-1 | c��H2��/mol•L-1 | c��CH3OH��/mol•L-1 |
| ��ʼ | 1 | 3 | 0 |
| ��2min | 0.8 | 2.6 | 0.2 |
| ��4min | 0.4 | 1.8 | 0.6 |
| ��6min | 0.4 | 1.8 | 0.6 |
| A�� | ��4 min����6 min�û�ѧ��Ӧ����ƽ��״̬ | |
| B�� | ��2 minʱ�����ֻ�ı�ijһ��������ı���������ܼ�����H2 | |
| C�� | ��2 minʱ�����ֻ�ı�ijһ��������ı������������ʹ�ô��� | |
| D�� | ��6 minʱ�������������䣬��������¶ȣ�����Ӧ�������� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | FeO�۳� | B�� | Fe3O4�۳� | C�� | Fe2O3�۳� | D�� | NO2���� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com