
”¾ĢāÄæ”æA”¢B”¢C”¢D”¢EŹĒŌ×ÓŠņŹżŅĄ“ĪµŻŌöµÄĪåÖÖ³£¼ūŌŖĖŲ”£A”¢BŌŖĖŲ×é³ÉµÄĘųĢ¬»ÆŗĻĪļMµÄĖ®ČÜŅŗ³Ź¼īŠŌ£¬CŌŖĖŲŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ£¬DµÄµ„ÖŹŌŚCµÄµ„ÖŹÖŠČ¼ÉÕŗóµÄ²śĪļæÉŅŌŹ¹Ę·ŗģČÜŅŗĶŹÉ«£¬EŹĒ½šŹōŌŖĖŲ”£
(1)Š“³öA”¢CĮ½ÖÖŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļA2C2µÄµē×ÓŹ½_______”£
(2)Čō½«E½šŹōĶ¶ČėŃĪĖįÖŠ£¬Éś³ÉĮĖĒ³ĀĢÉ«ČÜŅŗ£Ī”£Ōņ£ĪµÄĖįŠŌČÜŅŗÓėA2C2·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ______________________________________”£
(3)RŹĒBµÄŃõ»ÆĪļ£¬Ķس£ĒéæöĻĀ³Źŗģ×ŲÉ«”£ĻÖÓŠŅ»ŹŌ¹ÜR£¬ÓūŹ¹ŌŖĖŲBČ«²æ×Ŗ»ÆĪŖĖüµÄ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦µÄĖ®»ÆĪļ£¬¹Ź½ųŠŠČēĻĀŹµŃé²Ł×÷£ŗ½«Ź¢ÓŠRµÄŹŌ¹Üµ¹æŪŌŚĖ®²ŪÖŠ£¬______”£
(4)ÓŠČĖÉčĻėŃ°ĒóŗĻŹŹµÄ“߻ƼĮŗĶµē¼«²ÄĮĻ£¬ŅŌA2”¢B2ĪŖµē¼«·“Ó¦Īļ£¬ŅŌHCl”ŖNH4ClČÜŅŗĪŖµē½āÖŹČÜŅŗÖĘŌģŠĀŠĶČ¼ĮĻµē³Ų£¬ŹŌŠ“³öøƵē³ŲµÄÕż¼«·“Ó¦Ź½_____________£¬·ÅµēŹ±£¬ČÜŅŗÖŠµÄH+ŅĘĻņ_________(ĢīÕż¼«»ņøŗ¼«)”£
”¾“š°ø”æ![]() 2Fe2+ + 2H+ +H2O2 = 2Fe3+ +2H2O ĻņŹŌ¹ÜÖŠ»ŗ»ŗĶØČė×ćĮæŃõĘų N2 +8H+ +6e- =2NH
2Fe2+ + 2H+ +H2O2 = 2Fe3+ +2H2O ĻņŹŌ¹ÜÖŠ»ŗ»ŗĶØČė×ćĮæŃõĘų N2 +8H+ +6e- =2NH![]() Õż¼«
Õż¼«
”¾½āĪö”æ
A”¢B”¢C”¢D”¢EŹĒŌ×ÓŠņŹżŅĄ“ĪµŻŌöµÄĪåÖÖ³£¼ūŌŖĖŲ”£CŌŖĖŲŹĒµŲæĒÖŠŗ¬Įæ×ī¶ąµÄŌŖĖŲ£¬ŌņCĪŖOŌŖĖŲ£»A”¢B×é³ÉµÄĘųĢ¬»ÆŗĻĪļMµÄĖ®ČÜŅŗ³Ź¼īŠŌ£¬æÉĶĘÖŖBĪŖNŌŖĖŲ”¢AĪŖHŌŖĖŲ£¬MĪŖNH3£»DµÄµ„ÖŹŌŚO2ÖŠČ¼ÉÕ²śĪļæÉŹ¹Ę·ŗģČÜŅŗĶŹÉ«£¬æÉĶĘÖŖDĪŖSŌŖĖŲ”£
(1) A”¢CĮ½ÖÖŌŖĖŲ×é³ÉµÄ»ÆŗĻĪļA2C2ĪŖH2O2£¬ŹōÓŚ¹²¼Ū»ÆŗĻĪļ£¬Ęäµē×ÓŹ½ĪŖ![]() £»
£»
(2) EŹĒ½šŹōŌŖĖŲ£¬½«E½šŹōĶ¶Čėµ½ŃĪĖįČÜŅŗÖŠ£¬Éś³ÉĮĖĒ³ĀĢÉ«ČÜŅŗN£¬ŌņEĪŖFeŌŖĖŲ£¬NĪŖFeCl2”£Fe2+¾ßÓŠ»¹ŌŠŌ£¬H2O2¾ßÓŠŃõ»ÆŠŌ£¬¹Ź¶žÕß·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ2Fe2+ + 2H+ +H2O2 = 2Fe3+ +2H2O£»
(3)RŹĒBµÄŃõ»ÆĪļ£¬Ķس£ĒéæöĻĀ³Źŗģ×ŲÉ«£¬ŌņRĪŖNO2£¬Óū½«NO2Č«²æ×Ŗ»ÆĪŖHNO3£¬½«Ź¢ÓŠNO2µÄŹŌ¹Üµ¹æŪŌŚĖ®²ŪÖŠ»¹ŠčĻņŹŌ¹ÜÖŠ»ŗ»ŗĶØČė×ćĮæŃõĘų£»
(4) ŅŌH2”¢N2ĪŖµē¼«·“Ó¦Īļ£¬ŅŌHCl”ŖNH4ClČÜŅŗĪŖµē½āÖŹČÜŅŗÖĘŌģŠĀŠĶČ¼ĮĻµē³Ų£¬øł¾ŻH2ÓėN2ŗĻ³É°±ŌĄķæÉÖŖ£¬H2Ź§µē×Ó£¬“Óøŗ¼«ĶØČė£¬N2µĆµē×Ó£¬“ÓÕż¼«ĶØČė£¬¹ŹÕż¼«·“Ó¦Ź½ĪŖN2 +8H+ +6e- =2NH![]() £¬·ÅµēŹ±£¬ČÜŅŗÖŠµÄH+ĪŖŃōĄė×Ó£¬Ó¦ŅĘĻņÕż¼«”£
£¬·ÅµēŹ±£¬ČÜŅŗÖŠµÄH+ĪŖŃōĄė×Ó£¬Ó¦ŅĘĻņÕż¼«”£



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æÉčNAĪŖ°¢·üŁ¤µĀĀŽµÄÖµ£¬ ŅŃÖŖHClĘųĢåµÄÖŹĮæĪŖ3.65g
(1)HClµÄĪļÖŹµÄĮæĪŖ_______________
(2)HClµÄ·Ö×ÓøöŹżĪŖ_______________
(3)Ō×Ó×ÜŹżĪŖ _______________
(4)ŌŚ±ź×¼×“æöµÄĢå»żĪŖ________________
(5)Ėłŗ¬µē×ÓŹżĪŖ _________________
(6)Čē°Ń HClĶźČ«ČÜÓŚĖ®ÅäÖĆ³É1L ČÜŅŗ£¬øĆČÜŅŗÖŠH+µÄĪļÖŹµÄĮæÅضČĪŖ_________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ°“ŅŖĒó»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ĢžAŌŚĶ¬ĪĀ”¢Ķ¬Ń¹ĻĀÕōĘųµÄĆܶȏĒH2µÄ35±¶£¬Ęä·Ö×ÓŹ½ĪŖ________”£
£Ø2£©3”Ŗ¼×»ł”Ŗ1Ņ»¶”Č²µÄ½į¹¹¼ņŹ½ĪŖ________
£Ø3£©![]() µÄ·Ö×ÓŹ½ĪŖ__________
µÄ·Ö×ÓŹ½ĪŖ__________
£Ø4£©·Ö×ÓŹ½ĪŖC8H10ŹōÓŚ·¼ĻćĢžµÄĶ¬·ÖŅģ¹¹Ģå¹²ÓŠ______ÖÖ£¬ĘäÖŠ_______£Ø½į¹¹¼ņŹ½£©ŌŚ±½»·ÉĻµÄŅ»ĀČČ”“ś²śĪļÓŠĮ½ÖÖ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æ»Æѧ·“Ó¦A2+B2 = 2ABµÄÄÜĮæ±ä»ÆČēĶ¼ĖłŹ¾£¬ŌņĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
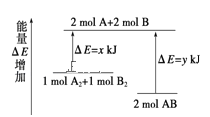
A.øĆ·“Ó¦ŹĒĪüČČ·“Ó¦
B.¶ĻĮŃ 1molA-A ¼üŗĶ 1molB-B ¼üÄܷųöxkJ µÄÄÜĮæ
C.2mol AB µÄ×ÜÄÜĮæøßÓŚ1mol A2ŗĶ1mol B2ŗĶµÄ×ÜÄÜĮæ
D.¶ĻĮŃ 2mol A-B ¼üŠčŅŖĪüŹÕykJ µÄÄÜĮæ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æŗ£ŃóŹĒŅ»øöŌ¶Ī“ĶźČ«æŖ·¢µÄ¾Ž“ó»Æѧ׏Ō“±¦æā”£
(1)ĪŽŠč¾¹ż»Æѧ±ä»Æ¾ĶÄÜ“Óŗ£Ė®ÖŠ»ńµĆµÄĪļÖŹŹĒ________£ØĢīŠņŗÅ£©
A ĀČĘų B µĖ® CÉÕ¼ī D Ź³ŃĪ
(2)“Óŗ£Ė®ÖʵƵēÖŃĪÖŠŗ¬ÓŠ½Ļ¶ąµÄMg2+”¢Ca2+”¢SO![]() µČ£¬ŅŖ³żČ„ÕāŠ©Ąė×Ó£¬ĻĀĮŠ¼ÓČėŅ©Ę·Ė³ŠņÕżČ·µÄŹĒ________£ØĢīŠņŗÅ£©
µČ£¬ŅŖ³żČ„ÕāŠ©Ąė×Ó£¬ĻĀĮŠ¼ÓČėŅ©Ę·Ė³ŠņÕżČ·µÄŹĒ________£ØĢīŠņŗÅ£©
A NaOHČÜŅŗ”śNa2CO3ČÜŅŗ”śBaCl2ČÜŅŗB BaCl2ČÜŅŗ”śNaOHČÜŅŗ”śNa2CO3ČÜŅŗ
C NaOHČÜŅŗ”śBaCl2ČÜŅŗ”śNa2CO3ČÜŅŗD Na2CO3ČÜŅŗ ”śNaOHČÜŅŗ”ś BaCl2ČÜŅŗ
(3)“Óŗ£Ė®ÖŠµĆµ½äåµÄ¹ż³ĢČēĻĀ£ŗ
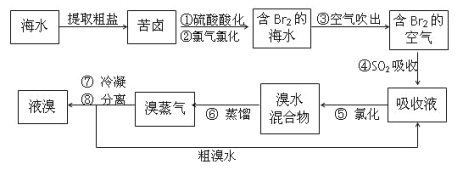
¢ŁŠ“³öÓÉ”°æąĀ±”±±ä³É”°ŗ¬äåŗ£Ė®”±µÄĄė×Ó·½³ĢŹ½________
¢ŚŠ“³ö¢Ü·¢Éś·“Ó¦µÄ»Æѧ·½³ĢŹ½________
¢Ūij»ÆѧŠ”×éµÄĶ¬Ń§ĪŖĮĖĮĖ½ā“Ó¹¤ŅµäåÖŠĢį“æäåµÄ·½·Ø£¬²éŌÄĮĖÓŠ¹Ų׏ĮĻ£ŗBr2µÄ·ŠµćĪŖ59 ”ę£¬Ī¢ČÜÓŚĖ®£¬ÓŠ¶¾ŠŌŗĶĒæøÆŹ“ŠŌ”£ĖūĆĒ²Ī¹ŪÉś²ś¹ż³Ģŗó£¬Éč¼ĘĮĖČēĶ¼ĖłŹ¾ŹµŃé×°ÖĆ£ŗ
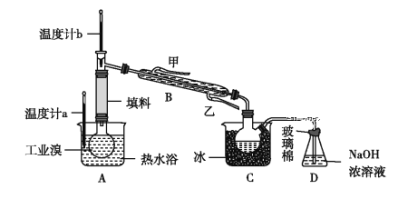
Ķ¼ÖŠŅĒĘ÷BĄäČ“Ė®µÄ³öæŚĪŖ______(Ģī”°¼×”±»ņ”°ŅŅ”±)£¬D×°ÖƵÄ×÷ÓĆŹĒ__________£¬·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ__________£¬ÕūĢ׏µŃé×°ÖĆÖŠŅĒĘ÷Į¬½Ó¾ł²»ÄÜÓĆĻš½ŗČūŗĶĻš½ŗ¹Ü£¬ĘäŌŅņŹĒ__________”£
(4)ŅŃÖŖijČÜŅŗÖŠCl-”¢Br-”¢I-µÄĪļÖŹµÄĮæÖ®±ČĪŖ2£ŗ3£ŗ4£¬ĻÖÓūŹ¹ČÜŅŗÖŠµÄCl-”¢Br-”¢I-µÄĪļÖŹµÄĮæÖ®±ČĪŖ±äĪŖ4£ŗ3£ŗ2£¬ÄĒĆ“ĶØČėCl2µÄĪļÖŹµÄĮæŹĒŌČÜŅŗÖŠI-µÄĪļÖŹµÄĮæµÄ__________
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”沬”¢īÜ”¢Äų¼°Ęä»ÆŗĻĪļŌŚ¹¤ŅµŗĶŅ½Ņ©µČĮģÓņÓŠÖŲŅŖÓ¦ÓĆ”£»Ų“šĻĀĮŠĪŹĢā:
(1)Öž²Ø²ÄĮĻæĘѧ¹ś¼ŅŹµŃéŹŅæĘŃŠŠ”×é·¢ĻÖĮĖŌŚ5KĻĀ³ŹĻÖ³¬µ¼ŠŌµÄ¾§ĢåCoO2£¬øĆ¾§Ģå¾ßÓŠ²ćד½į¹¹”£
¢Ł¾§ĢåÖŠŌ×ÓCoÓėOµÄÅäĪ»ŹżÖ®±ČĪŖ_________”£
¢Ś»łĢ¬īÜŌ×ӵļŪµē×ÓÅŲ¼Ķ¼ĪŖ_______”£
(2)ÅäŗĻĪļNi(CO)4³£ĪĀĻĀĪŖŅŗĢ¬£¬Ņ×ČÜÓŚCCl4”¢±½µČÓŠ»śČܼĮ”£¹ĢĢ¬Ni(CO)4ŹōÓŚ_____¾§Ģ壻Š“³öĮ½ÖÖÓėCO¾ßÓŠĻąĶ¬æռ乹ŠĶŗĶ¼üŗĻŠĪŹ½µÄ·Ö×Ó»ņĄė×Ó£ŗ_______”£
(3)ijÄųÅäŗĻĪļ½į¹¹ČēĶ¼ĖłŹ¾:
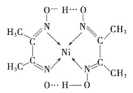
¢Ł·Ö×ÓÄŚŗ¬ÓŠµÄ»Æѧ¼üÓŠ___________(ĢīŠņŗÅ).
A Ēā¼ü B Ąė×Ó¼ü C ¹²¼Ū¼ü D ½šŹō¼ü E ÅäĪ»¼ü
¢ŚÅäŗĻĪļÖŠC”¢N”¢OČżÖÖŌŖĖŲŌ×ӵĵŚŅ»µēĄėÄÜÓɓ󵽊”µÄĖ³ŠņŹĒN> O>C£¬ŹŌ“ÓŌ×Ó½į¹¹½āŹĶĪŖŹ²Ć“Ķ¬ÖÜĘŚŌŖĖŲŌ×ӵĵŚŅ»µēĄėÄÜN>O_________”£
(4)Ä³ŃŠ¾æŠ”×é½«Ę½ĆęŠĶµÄ²¬ÅäŗĻĪļ·Ö×Ó½ųŠŠ²ćד¶ŃĘö£¬Ź¹Ćæøö·Ö×ÓÖŠµÄ²¬Ō×ÓŌŚÄ³Ņ»·½ĻņÉĻÅÅĮŠ³ÉŠŠ£¬¹¹³ÉÄܵ¼µēµÄ”°·Ö×Ó½šŹō" £¬Ęä½į¹¹ČēĶ¼ĖłŹ¾”£

¢Ł"·Ö×Ó½šŹō"æÉŅŌµ¼µē£¬ŹĒŅņĪŖ______ÄÜŃŲ×ÅĘäÖŠµÄ½šŹōŌ×ÓĮ“Į÷¶Æ”£
¢Ś"·Ö×Ó½šŹō"ÖŠ£¬²¬Ō×ÓŹĒ·ńŅŌsp3µÄ·½Ź½ŌÓ»Æ?_________(Ģī”°ŹĒ"»ņ”°·ń")£¬ĘäĄķÓÉŹĒ__________”£
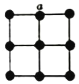
(5)½šŹō²¬¾§ĢåÖŠ£¬²¬Ō×ÓµÄÅäĪ»ŹżĪŖ12£¬ĘäĮ¢·½¾§°ūŃŲx”¢y»ņzÖįµÄĶ¶Ó°Ķ¼ČēĶ¼ĖłŹ¾£¬Čō½šŹō²¬µÄĆܶČĪŖd g”¤cm-3£¬Ōņ¾§°ū²ĪŹża=_______nm(ĮŠ¼ĘĖćŹ½)”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æX”¢Y”¢Z”¢WĪŖĖÄÖÖ¶ĢÖÜĘŚÖ÷×åŌŖĖŲ£¬X”¢YŌŚÖÜĘŚ±ķÖŠµÄĻą¶ŌĪ»ÖĆČēĶ¼ĖłŹ¾£¬Xn”¢Yn+”¢Z+¾ßÓŠĻąĶ¬µÄµē×Ó²ć½į¹¹£¬WµÄ×īÄŚ²ćµē×ÓŹżÓė×īĶā²ćµē×ÓŹżÖ®ŗĶµČÓŚ“ĪĶā²ćµē×ÓŹż”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ

A. Ō×Ó°ė¾¶£ŗr(X)<r(Y)<r(Z)<r(W)
B. XŠĪ³ÉµÄŃõ»ÆĪļµÄÖÖĄą×ī¶ąĪŖ4ÖÖ
C. ×īøß¼ŪŃõ»ÆĪļ¶ŌÓ¦Ė®»ÆĪļµÄ¼īŠŌ£ŗZ<Y
D. Y”¢Z”¢W¶ŌÓ¦µÄ×īøß¼ŪŃõ»ÆĪļµÄĖ®»ÆĪļÖ®¼äÄܹ»Į½Į½Ļą»„·“Ó¦
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”æĮøŹ³²Ö“¢³£ÓĆĮ×»ÆĀĮ(A1P)ѬÕōɱ³ę£¬A1PÓöĖ®¼“²śÉśĒ滹ŌŠŌµÄPH3ĘųĢ唣¹ś¼Ņ±ź×¼¹ę¶ØĮøŹ³ÖŠĮ×Īļ(ŅŌPH3¼Ę)µÄ²ŠĮōĮæ²»³¬¹ż0.05 mgkg-1Ź±ĪŖŗĻøń”£Ä³Š”×éĶ¬Ń§ÓĆČēĶ¼ĖłŹ¾ŹµŃé×°ÖĆŗĶŌĄķ²ā¶ØijĮøŹ³ŃłĘ·ÖŠµś»ÆĪļµÄ²ŠĮōĮ攣CÖŠ¼ÓČė100 gŌĮø£¬E ÖŠ¼ÓČė20.00mL2.50”ĮlO-4molL-1KMnO4ČÜŅŗµÄH2SO4Ėį»Æ)£¬CÖŠ¼ÓČė×ćĮæĖ®£¬³ä·Ö·“Ó¦ŗó£¬ÓĆŃĒĮņĖįÄʱź×¼ČÜŅŗµĪ¶ØEÖŠµÄČÜŅŗ”£
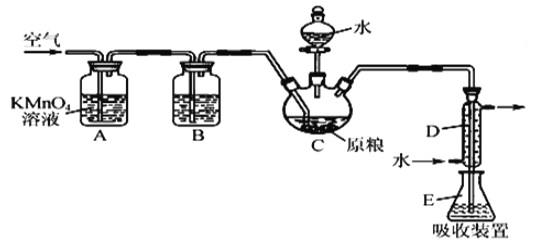
(1)×°ÖĆAÖŠµÄKMn04ČÜŅŗµÄ×÷ÓĆŹĒ_____”£
(2)×°ÖĆBÖŠŹ¢×°½¹ŠŌĆ»Ź³×ÓĖįµÄ¼īŠŌČÜŅŗĪüŹÕæÕĘųÖŠµÄO2”£ČōČ„µōøĆ×°ÖĆ£¬Ōņ²āµĆµÄĮ×»ÆĪļµÄ²ŠĮōĮæ___(Ģī”°Ę«ó{”±”°Ę«µĶ”±»ņ”°²»±ä”±)”£
(3)×°ÖĆEÖŠPH3Ńõ»Æ³ÉĮ×Ėį£¬MnO4-±»»¹ŌĪŖMn2+£¬Š“³öøĆ·“Ó¦µÄĄė×Ó·½³ĢŹ½£ŗ__________”£
(4)ŹÕ¼Æ×°ÖĆEÖŠµÄĪüŹÕŅŗ£¬¼ÓĖ®Ļ”ŹĶÖĮ250 mL£¬ĮæČ”ĘäÖŠµÄ25.00 mLӌ׶ŠĪĘæÖŠ£¬ ÓĆ4.0”ĮlO-5molL-1µÄNa2SO3±ź×¼ČÜŅŗµĪ¶Ø£¬ĻūŗÄNa2SO3±ź×¼ČÜŅŗ20.00mL£¬·“Ó¦ŌĄķŹĒ S02-+Mn04-+H+”śS042-+Mn2++H20(Ī“ÅäĘ½)Ķعż¼ĘĖćÅŠ¶ĻøĆѳʷŹĒ·ńŗĻøń(Š“³ö¼ĘĖć¹ż³Ģ)_______”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
”¾ĢāÄæ”涼ÄÜÓĆĻĀĶ¼ĖłŹ¾×°ÖĆ½ųŠŠÅēČŖŹµŃéµÄŅ»×éĘųĢåŹĒ
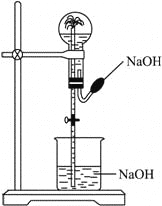
A.HClŗĶCO2B.NH3ŗĶCH4C.SO2ŗĶCOD.NO2ŗĶNO
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com