
ЁОЬтФПЁПЙЄвЕЩЯРћгУЗЯФјДпЛЏМС(жївЊГЩЗжЮЊNiЃЌЛЙКЌгавЛЖЈСПЕФZnЁЂFeЁЂSiO2ЁЂCaOЕШ)жЦБИВнЫсФјОЇЬхЕФСїГЬШчЯТЃК
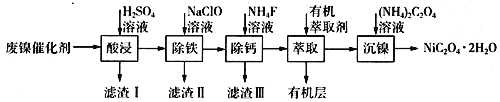
(1)ЧыаДГівЛжжФмЬсИпЁАЫсНўЁБЫйТЪЕФДыЪЉЃК________________________ЃЛТЫдќIЕФГЩЗжЪЧ____________(ЬюЛЏбЇЪН)ЁЃ
(2)Г§ЬњЪБЃЌПижЦВЛЭЌЕФЬѕМўПЩвдЕУЕНВЛЭЌЕФТЫдќIIЁЃвбжЊТЫдќIIЕФГЩЗжгыЮТЖШЁЂpHЕФЙиЯЕШчЭМЫљЪОЃК
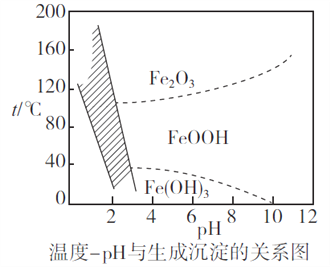
ЂйШєПижЦЮТЖШ40ЁцЁЂpH=8ЃЌдђТЫдќIIЕФжївЊГЩЗжЮЊ_________________________(ЬюЛЏбЇЪН)ЁЃ
ЂкШєПижЦЮТЖШ80ЁцЁЂpH=2ЃЌПЩЕУЕНЛЦЬњЗЏФЦ[Na2Fe6(SO4)4(OH)12](ЭМжавѕгАВПЗж)ЃЌаДГіЩњГЩЛЦЬњЗЏФЦЕФРызгЗНГЬЪНЃК___________________________________________ЁЃ
(3)вбжЊГ§ЬњКѓЫљЕУ100 mLШмвКжаc(Ca2+)=0.01molЁЄL-1ЃЌМгШы100 mL NH4FШмвКЃЌЪЙCa2+ЧЁКУГСЕэЭъШЋМДШмвКжаc(Ca2+)=1ЁС10-5 molЁЄL-1ЃЌдђЫљМгc(NH4F)=_________molЁЄL-1ЁЃ[вбжЊKsp(CaF2)=5.29ЁС10-9]
(4)МгШыгаЛњнЭШЁМСЕФзїгУЪЧ________________________ЁЃ
(5)ФГЛЏбЇЖЦФјЪдМСЕФЛЏбЇЪНЮЊMxNi(SO4)y(MЮЊ+1МлбєРызгЃЌNiЮЊ+2МлЃЌxЁЂyОљЮЊе§ећЪ§)ЁЃЮЊВтЖЈИУЖЦФјЪдМСЕФзщГЩЃЌНјааШчЯТЪЕбщЃК
IЃЎГЦСП28.7gЖЦФјЪдМСЃЌХфжЦ100 mLШмвКAЃЛ
ЂђЃЎзМШЗСПШЁ10.00 mLШмвКAЃЌгУ0.40 molЁЄL-1ЕФEDTA(Na2H2Y)БъзМШмвКЕЮЖЈЦфжаЕФNi2+(РызгЗНГЬЪНЮЊNi2++H2Y2-=NiY2-+2H+)ЃЌЯћКФEDTAБъзМШмвК25.00mLЃЛ
ЂѓЃЎСэШЁ10.00 mLШмвКAЃЌМгШызуСПЕФBaCl2ШмвКЃЌЕУЕНАзЩЋГСЕэ4.66gЁЃ
ЂйХфжЦ100 mLЖЦФјЪдМСЪБЃЌашвЊЕФвЧЦїГ§вЉГзЁЂЭаХЬЬьЦНЁЂВЃСЇАєЁЂЩеБЁЂСПЭВЁЂНКЭЗЕЮЙмЭтЃЌЛЙашвЊ________________________ЁЃ
ЂкИУЖЦФјЪдМСЕФЛЏбЇЪНЮЊ________________________________ЁЃ
ЁОД№АИЁП АбЗЯФјДпЛЏМСЗлЫщЁЂЪЪЕБМгШШЃЌЪЪЕБдіДѓЫсЕФХЈЖШЛђНСАшЕШ SiO2ЁЂCaSO4 FeOOH 2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12Ё§+3Cl-+6H+ 6.6ЁС10-2 Г§ШЅШмвКжаЕФZn2+ 100mLШнСПЦП (NH4)2Ni(SO4)2
ЁОНтЮіЁПЪдЬтЗжЮіЃК(1)ИљОнгАЯьЗДгІЫйТЪЕФвђЫиЗжЮіЬсИпЁАЫсНўЁБЫйТЪЕФДыЪЉЃЛЗЯФјДпЛЏМСжаSiO2гыСђЫсВЛЗДгІЃЌCaOгыСђЫсЗДгІЕФВњЮяCaSO4ЮЂШмгкЫЎЃЛ(2)ИљОнТЫдќIIЕФГЩЗжгыЮТЖШЁЂpHЕФЙиЯЕЭМЃЌПЩжЊПижЦЮТЖШ40ЁцЁЂpH=8ЪБЃЌТЫдќIIЕФжївЊГЩЗжЃЛЂкNa2Fe6(SO4)4(OH)12жаЬњдЊЫиЛЏКЯМлЪЧ+3ЃЌПЩжЊClO-АбFe2+бѕЛЏЮЊFe3+ЃЌЭЌЪБЩњГЩNa2Fe6(SO4)4(OH)12ГСЕэЃЛ(3)ИљОнЗНГЬЪНCa2++2F-= CaF2Ё§ЃЌГСЕэCa2+ЯћКФ0.002mol NH4F ЃЌИљОнKsp(CaF2)=5.29ЁС10-9ЃЌГСЕэCa2+КѓЃЌШмвКжаc(F-)=![]() ЃЛ(4)ИљОнСїГЬЭМЃЌМгШыгаЛњнЭШЁМСЕФзїгУЪЧГ§ШЅШмвКжаЕФZn2+ЃЛ(5)ЂйИљОнХфжЦ100mLвЛЖЈЮяжЪЕФСПХЈЖШШмвКЗжЮіашвЊЕФвЧЦїЃЛЂкИљОнNi2++H2Y2-=NiY2-+2H+МЦЫуNi2+ЕФЮяжЪЕФСПЃЌИљОн
ЃЛ(4)ИљОнСїГЬЭМЃЌМгШыгаЛњнЭШЁМСЕФзїгУЪЧГ§ШЅШмвКжаЕФZn2+ЃЛ(5)ЂйИљОнХфжЦ100mLвЛЖЈЮяжЪЕФСПХЈЖШШмвКЗжЮіашвЊЕФвЧЦїЃЛЂкИљОнNi2++H2Y2-=NiY2-+2H+МЦЫуNi2+ЕФЮяжЪЕФСПЃЌИљОн![]() ПЩМЦЫуЯрЖдЗжзгжЪСПЃЛИљОн10.00 mLШмвКAЃЌМгШызуСПЕФBaCl2ШмвКЃЌЕУЕНАзЩЋГСЕэ4.66gЃЌПЩМЦЫуSO42-ЕФЮяжЪЕФСПЃЌИљОнn(Ni2+)ЃКn(SO42-)МЦЫуyжЕЃЌИљОнЛЏКЯМлДњЪ§КЭЕШгкСуМЦЫуxжЕЃЌзюКѓИљОнЯрЖдЗжзгжЪСПМЦЫуMЕФЯрЖддзгжЪСПЁЃ
ПЩМЦЫуЯрЖдЗжзгжЪСПЃЛИљОн10.00 mLШмвКAЃЌМгШызуСПЕФBaCl2ШмвКЃЌЕУЕНАзЩЋГСЕэ4.66gЃЌПЩМЦЫуSO42-ЕФЮяжЪЕФСПЃЌИљОнn(Ni2+)ЃКn(SO42-)МЦЫуyжЕЃЌИљОнЛЏКЯМлДњЪ§КЭЕШгкСуМЦЫуxжЕЃЌзюКѓИљОнЯрЖдЗжзгжЪСПМЦЫуMЕФЯрЖддзгжЪСПЁЃ
НтЮіЃК(1)ИљОнгАЯьЗДгІЫйТЪЕФвђЫиЃЌЩ§ИпЮТЖШЁЂАбЗЯФјДпЛЏМСЗлЫщЁЂЪЪЕБдіДѓЫсЕФХЈЖШЛђНСАшЕШЃЌЖМПЩвдЬсИпЁАЫсНўЁБЫйТЪЃЛЗЯФјДпЛЏМСжаSiO2гыСђЫсВЛЗДгІЃЌCaOгыСђЫсЗДгІЕФВњЮяCaSO4ЮЂШмгкЫЎЃЌЫљвдТЫдќIЕФГЩЗжЪЧSiO2ЁЂCaSO4ЃЛ(2) ЂйИљОнТЫдќIIЕФГЩЗжгыЮТЖШЁЂpHЕФЙиЯЕЭМЃЌПЩжЊПижЦЮТЖШ40ЁцЁЂpH=8ЪБЃЌТЫдќIIЕФжївЊГЩЗжЪЧFeOOHЃЛЂкNa2Fe6(SO4)4(OH)12жаЬњдЊЫиЛЏКЯМлЪЧ+3ЃЌПЩжЊClO-АбFe2+бѕЛЏЮЊFe3+ЃЌЭЌЪБЩњГЩNa2Fe6(SO4)4(OH)12ГСЕэЃЌЗДгІЕФРызгЗНГЬЪНЪЧ2Na++3ClO-+6Fe2++4SO42-+9H2O=Na2Fe6(SO4)4(OH)12Ё§+3Cl-+6H+ЃЛ(3)ИљОнЗНГЬЪНCa2++2F-= CaF2Ё§ЃЌГСЕэCa2+ЯћКФ0.002mol NH4F ЃЌИљОнKsp(CaF2)=5.29ЁС10-9ЃЌГСЕэCa2+КѓЃЌШмвКжаc(F-)=![]() ЃЌЩшМгШыc(NH4F)=c molЁЄL-1ЃЌдђ
ЃЌЩшМгШыc(NH4F)=c molЁЄL-1ЃЌдђ![]() =
=![]() ЃЛc=6.6ЁС10-2ЃЛ(4)ИљОнСїГЬЭМЃЌМгШыгаЛњнЭШЁМСЕФзїгУЪЧГ§ШЅШмвКжаЕФZn2+ЃЛ(5)ЂйИљОнХфжЦ100mLвЛЖЈЮяжЪЕФСПХЈЖШШмвКашвЊ100mLШнСПЦПЃЛЂкИљОнNi2++H2Y2-=NiY2-+2H+ЃЌn(Ni2+)=0.025L
ЃЛc=6.6ЁС10-2ЃЛ(4)ИљОнСїГЬЭМЃЌМгШыгаЛњнЭШЁМСЕФзїгУЪЧГ§ШЅШмвКжаЕФZn2+ЃЛ(5)ЂйИљОнХфжЦ100mLвЛЖЈЮяжЪЕФСПХЈЖШШмвКашвЊ100mLШнСПЦПЃЛЂкИљОнNi2++H2Y2-=NiY2-+2H+ЃЌn(Ni2+)=0.025L![]() 0.4 molЁЄL-1=0.01mol ЃЌИљОн
0.4 molЁЄL-1=0.01mol ЃЌИљОн ![]() ЃЌMxNi(SO4)yЕФЯрЖдЗжзгжЪСП=
ЃЌMxNi(SO4)yЕФЯрЖдЗжзгжЪСП=![]() ЃЛ 10.00 mLШмвКAЃЌМгШызуСПЕФBaCl2ШмвКЃЌЕУЕНСђЫсБЕ4.66gЃЌЫљвдSO42-ЕФЮяжЪЕФСП0.02molЃЌИљОнn(Ni2+)ЃКn(SO42-)=1ЃКyЃЌдђy=2ЃЌИљОнЛЏКЯМлДњЪ§КЭЕШгкСу,x=2ЃЌЩш MЕФЯрЖддзгжЪСПЪЧaЃЌдђ2a+59+96
ЃЛ 10.00 mLШмвКAЃЌМгШызуСПЕФBaCl2ШмвКЃЌЕУЕНСђЫсБЕ4.66gЃЌЫљвдSO42-ЕФЮяжЪЕФСП0.02molЃЌИљОнn(Ni2+)ЃКn(SO42-)=1ЃКyЃЌдђy=2ЃЌИљОнЛЏКЯМлДњЪ§КЭЕШгкСу,x=2ЃЌЩш MЕФЯрЖддзгжЪСПЪЧaЃЌдђ2a+59+96![]() =287ЃЛa=18ЃЌЫљвдMЪЧNH4+ЃЌИУЖЦФјЪдМСЕФЛЏбЇЪНЮЊ(NH4)2Ni(SO4)2ЁЃ
=287ЃЛa=18ЃЌЫљвдMЪЧNH4+ЃЌИУЖЦФјЪдМСЕФЛЏбЇЪНЮЊ(NH4)2Ni(SO4)2ЁЃ


 дФЖСПьГЕЯЕСаД№АИ
дФЖСПьГЕЯЕСаД№АИ
| ФъМЖ | ИпжаПЮГЬ | ФъМЖ | ГѕжаПЮГЬ |
| ИпвЛ | ИпвЛУтЗбПЮГЬЭЦМіЃЁ | ГѕвЛ | ГѕвЛУтЗбПЮГЬЭЦМіЃЁ |
| ИпЖў | ИпЖўУтЗбПЮГЬЭЦМіЃЁ | ГѕЖў | ГѕЖўУтЗбПЮГЬЭЦМіЃЁ |
| ИпШ§ | ИпШ§УтЗбПЮГЬЭЦМіЃЁ | ГѕШ§ | ГѕШ§УтЗбПЮГЬЭЦМіЃЁ |
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПФЩУзTiO2дкЭПСЯЁЂЙтДпЛЏЁЂЛЏзБЦЗЕШСьгђгазХМЋЦфЙуЗКЕФгІгУЁЃ
жЦБИФЩУзTiO2ЕФЗНЗЈжЎвЛЪЧTiCl4ЫЎНтЩњГЩTiO2xH2OЃЌОЙ§ТЫЁЂЫЎЯДГ§ШЅЦфжаЕФCl-ЃЌдйКцИЩЁЂБКЩеГ§ШЅЫЎЗжЕУЕНЗлЬхTiO2ЁЃ
гУбѕЛЏЛЙдЕЮЖЈЗЈВтЖЈTiO2ЕФжЪСПЗжЪ§ЃКвЛЖЈЬѕМўЯТЃЌНЋTiO2ШмНтВЂЛЙдЮЊTi3+ЃЌдйвдKSCNШмвКзїжИЪОМСЃЌгУNH4Fe(SO4)2БъзМШмвКЕЮЖЈTi3+жСШЋВПЩњГЩTi4+ЁЃ
ЧыЛиД№ЯТСаЮЪЬтЃК
ЃЈ1ЃЉTiCl4ЫЎНтЩњГЩTiO2xH2OЕФЛЏбЇЗНГЬЪНЮЊ______________________________ЁЃ
ЃЈ2ЃЉМьбщTiO2xH2OжаCl-ЪЧЗёБЛГ§ОЛЕФЗНЗЈЪЧ______________________________ЁЃ
ЃЈ3ЃЉХфжЦNH4Fe(SO4)2БъзМШмвКЪБЃЌМгШывЛЖЈСПH2SO4ЕФдвђЪЧ__________ЃЛЪЙгУЕФвЧЦїГ§ЬьЦНЁЂвЉГзЁЂВЃСЇАєЁЂЩеБЁЂСПЭВЭтЃЌЛЙашвЊЯТЭМжаЕФ__________ЃЈЬюзжФИДњКХЃЉЁЃ
ЃЈ4ЃЉЕЮЖЈжеЕуЕФХаЖЈЯжЯѓЪЧ________________________________________ЁЃ
ЃЈ5ЃЉЕЮЖЈЗжЮіЪБЃЌГЦШЁTiO2ЃЈФІЖћжЪСПЮЊMg/molЃЉЪдбљwgЃЌЯћКФcmol/LNH4Fe(SO4)2 БъзМШмвКVmLЃЌдђTiO2жЪСПЗжЪ§БэДяЪНЮЊ______________________________ЁЃ
ЃЈ6ЃЉХаЖЯЯТСаВйзїЖдTiO2жЪСПЗжЪ§ВтЖЈНсЙћЕФгАЯьЃЈЬюЁАЦЋИпЁБЁАЦЋЕЭЁБЛђЁАЮогАЯьЁБЃЉЁЃ
ЂйШєдкХфжЦБъзМШмвКЙ§ГЬжаЃЌЩеБжаЕФNH4Fe(SO4)2ШмвКгаЩйСПНІГіЃЌЪЙВтЖЈНсЙћ__________ЁЃ
ЂкШєдкЕЮЖЈжеЕуЖСШЁЕЮЖЈЙмПЬЖШЪБЃЌИЉЪгБъзМвКвКУцЃЌЪЙВтЖЈНсЙћ__________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЯТЭМЮЊжмЦкБэжаВПЗждЊЫиЕФФГжжаджЪ(XжЕ)ЫцдзгађЪ§БфЛЏЕФЙиЯЕЁЃ

(1)ЖЬжмЦкжадзгКЫЭтpЙьЕРЩЯЕчзгЪ§гыsЙьЕРЩЯЕчзгзмЪ§ЯрЕШЕФдЊЫиЪЧ________(аДдЊЫиЗћКХ)ЁЃ
(2)ЭЌжїзхФкВЛЭЌдЊЫиЕФXжЕБфЛЏЕФЬиЕуЪЧ ________________ЃЌЭЌжмЦкФкЃЌЫцзХдзгађЪ§ЕФдіДѓЃЌXжЕБфЛЏЕФзмЧїЪЦЪЧ________ЁЃжмЦкБэжаXжЕЕФетжжБфЛЏЬиЕуЬхЯжСЫдЊЫиаджЪЕФ____________БфЛЏЙцТЩЁЃ
(3)XжЕНЯаЁЕФдЊЫиМЏжадкдЊЫижмЦкБэЕФ________ЁЃ
aЃЎзѓЯТНЧЁЁ bЃЎгвЩЯНЧЁЁЁЁЁЁcЃЎЗжНчЯпИННќ
(4)ЯТСаЙигкдЊЫиИУаджЪЕФЫЕЗЈжае§ШЗЕФЪЧ________(бЁЬюДњКХ)ЁЃ
aЃЎXжЕПЩЗДгГдЊЫизюИпе§ЛЏКЯМлЕФБфЛЏЙцТЩ
bЃЎXжЕПЩЗДгГдзгдкЗжзгжаЮќв§ЕчзгЕФФмСІ
cЃЎXжЕДѓаЁПЩгУРДКтСПдЊЫиН№ЪєадКЭЗЧН№ЪєадЕФЧПШѕ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПгУЯТСазАжУНјааЯрЙиЪЕбщЃЌВйзїе§ШЗЧвФмДяЕНЪЕбщФПЕФЕФЪЧ
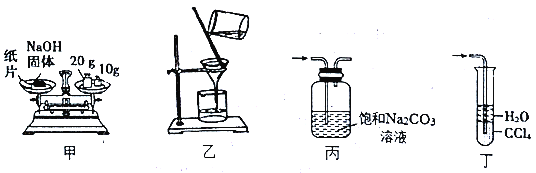
A. МззАжУ:ГЦ30.0gNaOHЙЬЬх B. ввзАжУ:Й§ТЫГ§ШЅШмвКжаЕФВЛШмаддгжЪ
C. БћзАжУ:Г§ШЅCO2жаЛьгаЕФHClЦјЬх D. ЖЁзАжУ:ЮќЪеЪЕбщЪвжЦNH3ЕФЮВЦј
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПбаОПЕЊМАЦфЛЏКЯЮяЕФаджЪЃЌПЩвдгааЇИФЩЦШЫРрЕФЩњДцЛЗОГЁЃЕЊдЊЫиЛЏКЯМлвЛЮяжЪРрБ№ЙиЯЕЭМШчЯТЁЃ
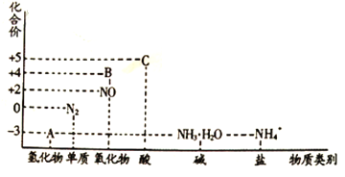
ЛиД№ЯТСаЮЪЬт:
ЃЈ1ЃЉдкДпЛЏМСКЭМгШШЕФЬѕМўЯТЃЌЮяжЪAЩњГЩNOЪЧЙЄвЕжЦЯѕЫсЕФживЊЗДгІЃЌЛЏбЇЗНГЬЪНЪЧ_______________________________________________________________________________ЁЃ
ЃЈ2ЃЉдкМгШШЬѕМўЯТЃЌЮяжЪCЕФХЈШмвКгыЬМЕЅжЪЗДгІЃЌаДГіЗДгІЕФЛЏбЇЗНГЬЪН______________ЁЃ
ЃЈ3ЃЉЪЕбщЪвжаЃЌМьбщШмвКжаКЌгаNH4+ЕФВйзїЗНЗЈЪЧ______________ЁЃ
ЃЈ4ЃЉЮяжЪBЮЊКьзиЩЋЦјЬхЃЌаДГіИУЮяжЪгыЫЎЗДгІЕФРызгЗНГЬЪН______________________ЃЌЕБЗДгІЯћКФ3.36L(БъзМзДПі)ЮяжЪBЪБЃЌзЊвЦЕчзгЕФЮяжЪЕФСПЮЊ______________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвЛЖЈСПЭгы100mLcmol/LЕФЯЁЯѕЫсЗДгІЃЌВњЩњ1.12LNO(БъзМзДПі)ЃЌЗДгІНсЪјКѓЃЌЯђЗДгІКѓЕФШмвКжаЕЮМг1.0mol/LЕФNaOHШмвКЃЌЕЮМгЙ§ГЬжаЃЌВњЩњГСЕэЕФжЪСПгыМгШыNaOH ШмвКЕФЬхЛ§ЙиЯЕШчЯТЭМЫљЪОЁЃ
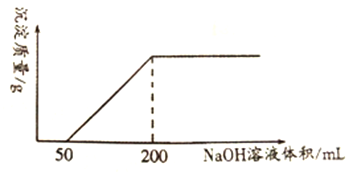
ЛиД№ЯТСаЮЪЬт:
ЃЈ1ЃЉаДГіЭгыЯЁЯѕЫсЗДгІЕФРызгЗНГЬЪН_____________________________________________ЁЃ
ЃЈ2ЃЉЭгыЯЁЯѕЫсЗДгІжаБЛЛЙдHNO3ЕФЮяжЪЕФСПЮЊ________molЁЃ
ЃЈ3ЃЉЭгыЯЁЯѕЫсЗДгІКѓЕФШмвКжаЃЌH+ЕФЮяжЪЕФСПХЈЖШЮЊ________mol/L(ЗДгІЧАКѓШмвКЬхЛ§БфЛЏКіТдВЛМЦ)ЁЃ
ЃЈ4ЃЉЯЁЯѕЫсЕФЮяжЪЕФСПХЈЖШc=______mol/LЁЃ
ЃЈ5ЃЉНЋЭгыЯЁЯѕЫсЗДгІЩњГЩЕФNOЭЈШыNaOH ШмвКжаЃЌВЂМгШы20%ЕФH2O2ЫЎШмвКЪЙЦфШЋВПзЊЛЏЮЊNaNO3ЃЌЗДгІЕФЛЏбЇЗНГЬЪНЪЧ______________________________________ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁП(1)CHЁЂCH3ЁЂCHЖМЪЧживЊЕФгаЛњЗДгІжаМфЬхЃЌЫќУЧЕФЕчзгЪНЗжБ№ЪЧ________ЁЂ________ЁЂ________ЃЛЦфжаCHжаЫФИідзгЪЧЙВЦНУцЕФЃЌШ§ИіМќНЧЯрЕШЃЌдђМќНЧгІЪЧ________ЁЃ
(2)ЕўЕЊЛЏКЯЮядкЛЏбЇЙЄвЕЩЯгаживЊгІгУЁЃNНазіЕўЕЊРызгЃЌЧыаДГі3жжгЩШ§ИідзгЙЙГЩЕФКЌгагыNЕФЕчзгЪ§ЯрЭЌЕФСЃзгЕФЛЏбЇЪН______ЁЂ______ЁЂ______ЁЃ
(3)NЁдNМќЕФМќФмЮЊ946 kJ/molЃЌNЁЊNМќЕФМќФмЮЊ193 kJ/molЃЌЫЕУїN2жаЕФ________МќБШ________МќЮШЖЈ(ЬюЁАІвЁБЛђЁАІаЁБ)
(4)CaC2жаC![]() гыO
гыO![]() ЛЅЮЊЕШЕчзгЬхЃЌO
ЛЅЮЊЕШЕчзгЬхЃЌO![]() ЕФЕчзгЪНПЩБэЪОЮЊ________________ЃЛ1 mol O
ЕФЕчзгЪНПЩБэЪОЮЊ________________ЃЛ1 mol O![]() жаКЌгаЕФІаМќЪ§ФПЮЊ________ЁЃ
жаКЌгаЕФІаМќЪ§ФПЮЊ________ЁЃ
(5)PH3дкГЃЮТЯТЪЧвЛжжЮоЩЋЁЂОчЖОЁЂвзздШМЕФЦјЬхЃЌЗжзгНсЙЙКЭNH3ЯрЫЦЁЃдкГЃЮТЯТ1ЬхЛ§ЕФЫЎФмШмНт0.26ЬхЛ§ЕФPH3ЃЌPH3КЭТБЛЏЧт(HX)зїгУЩњГЩЯргІЕФЛЏКЯЮяPH4XЃЌPH4XдкЫЎШмвКжаЭъШЋЫЎНт(PHНсЙЙРрЫЦгкCH4)ЁЃPH3ЕФЗжзгНсЙЙЕФаЮзДЪЧ________ЃЛдкPHжаPЃHМќжЎМфЕФМаНЧЪЧ_____ЁЃ
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПЬюаДЯТСаБэИё
ЮяжЪ | ЗжзгЪ§ | жЪСП(g) | ЮяжЪЕФСП(mol) | ФІЖћжЪСП(g/mol) |
ЕЊЦј | _________ | 14 | _________ | 28 |
ЫЎ | _________ | _________ | 2 | 18 |
ЯѕЫс | 9.03ЁС1023 | _________ | _________ | 63 |
ВщПДД№АИКЭНтЮі>>
ПЦФПЃКИпжаЛЏбЇ РДдДЃК ЬтаЭЃК
ЁОЬтФПЁПвдЯТСљжжЮяжЪЪЧвЛИібѕЛЏЛЙдЗДгІЕФЗДгІЮяКЭЩњГЩЮяЃКNOЁЂFeSO4ЁЂH2OЁЂFe(NO3)3ЁЂHNO3КЭFe2(SO4)3. аДГіИУЗДгІЗНГЬЪНВЂХфЦНЃЌгУЕЅЯпЧХБъГіЕчзгзЊвЦЗНЯђгыЪ§ФПЁЃ____________________ ЃЌИУЗДгІжаЃЌбѕЛЏМСЪЧ___________ЃЌБЛбѕЛЏЕФдЊЫиЪЧ___________ЃЌШєВњЩњБъзМзДПіЯТ11.2LЦјЬхЃЌзЊвЦЕчзгЪ§ЮЊ____________ЁЃ
ВщПДД№АИКЭНтЮі>>
АйЖШжТаХ - СЗЯАВсСаБэ - ЪдЬтСаБэ
КўББЪЁЛЅСЊЭјЮЅЗЈКЭВЛСМаХЯЂОйБЈЦНЬЈ | ЭјЩЯгаКІаХЯЂОйБЈзЈЧј | ЕчаХеЉЦОйБЈзЈЧј | ЩцРњЪЗащЮожївхгаКІаХЯЂОйБЈзЈЧј | ЩцЦѓЧжШЈОйБЈзЈЧј
ЮЅЗЈКЭВЛСМаХЯЂОйБЈЕчЛАЃК027-86699610 ОйБЈгЪЯфЃК58377363@163.com