
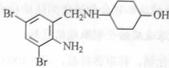
 £¬²»æ¼ĀĒĮ¢ĢåŅģ¹¹£©ŹĒĮŁ“²ÉĻŹ¹ÓĆ¹ć·ŗµÄ”£ĻĀĶ¼ĖłŹ¾µÄŹĒ¶ąĢõŗĻ³ÉĀ·ĻßÖŠµÄŅ»Ģõ£Ø·“Ó¦ŹŌ¼ĮŗĶ·“Ó¦Ģõ¼ž¾łĪ“±ź³ö£©
£¬²»æ¼ĀĒĮ¢ĢåŅģ¹¹£©ŹĒĮŁ“²ÉĻŹ¹ÓĆ¹ć·ŗµÄ”£ĻĀĶ¼ĖłŹ¾µÄŹĒ¶ąĢõŗĻ³ÉĀ·ĻßÖŠµÄŅ»Ģõ£Ø·“Ó¦ŹŌ¼ĮŗĶ·“Ó¦Ģõ¼ž¾łĪ“±ź³ö£©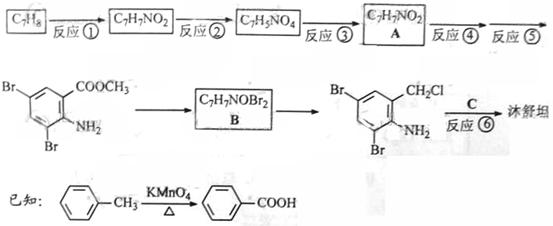
 £¬
£¬
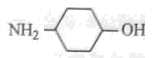 ÓėHCl·“Ӧɜ³ÉŃĪ
ÓėHCl·“Ӧɜ³ÉŃĪ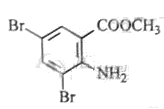 æÉČ·¶ØC7H8ĪŖ¼×±½£¬Ōņ·“Ó¦¢ŁĪŖ¼×±½ŌŚÅØĮņĖįŗĶ¼ÓČȵÄĢõ¼žĻĀÓėĻõĖį·¢ÉśČ”“ś·“Ó¦”£¼“
æÉČ·¶ØC7H8ĪŖ¼×±½£¬Ōņ·“Ó¦¢ŁĪŖ¼×±½ŌŚÅØĮņĖįŗĶ¼ÓČȵÄĢõ¼žĻĀÓėĻõĖį·¢ÉśČ”“ś·“Ó¦”£¼“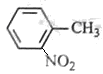
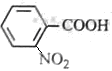
 A£ŗ
A£ŗ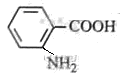 £¬·“Ó¦¢ŁČ”“ś£¬¢ŚŃõ»Æ£¬¢Ū»¹Ō£¬¢Ü¢ŻŹĒõ„»Æ”¢äå“ś£¬øł¾ŻBµÄ·Ö×ÓŹ½ŗĶ
£¬·“Ó¦¢ŁČ”“ś£¬¢ŚŃõ»Æ£¬¢Ū»¹Ō£¬¢Ü¢ŻŹĒõ„»Æ”¢äå“ś£¬øł¾ŻBµÄ·Ö×ÓŹ½ŗĶ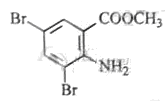 ”¢
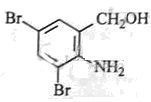
”¢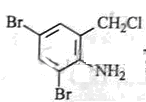 µÄ½į¹¹æÉÖŖBĪŖ
µÄ½į¹¹æÉÖŖBĪŖ £¬·“Ó¦¢ŽĪŖČ”“ś£¬CĪŖ
£¬·“Ó¦¢ŽĪŖČ”“ś£¬CĪŖ £¬·Ö×ÓŹ½ĪŖC6H13NO,¼ÓČėĢ¼Ėį¼ŲŹĒÖŠŗĶÉś³ÉµÄHCl£¬·ĄÖ¹·“Ó¦Īļ
£¬·Ö×ÓŹ½ĪŖC6H13NO,¼ÓČėĢ¼Ėį¼ŲŹĒÖŠŗĶÉś³ÉµÄHCl£¬·ĄÖ¹·“Ó¦Īļ ÓėHCl·“Ӧɜ³ÉŃĪ£¬²śĘ·²»“棬CµÄ²»±„ŗĶ¶ČĪŖ1£¬ĘäĶ¬·ÖŅģ¹¹ĢåæÉ·¢ÉśĖ®½ā·“Ó¦£¬Ōņŗ¬ėļü£¬ÓÖÓÉÓŚÖ»ŗ¬3ÖÖ²»Ķ¬»Æѧ»·¾³ĒāŌ×Ó£¬Ó¦øĆø߶ȶŌ³Ę£¬øł¾ŻÓŠŠņĖ¼Ī¬æÉŠ“³ö£ŗ(CH3)3CCONHCH3”¢(CH3)3CCH2CONH2”¢CH3CONHC£ØCH3£©3”¢HCON£ØCH3£©C£ØCH3£©3”¢CH3CON£ØCH2CH3£©2µČ”£- NH2ÓŠ»¹ŌŠŌ£¬Ņ×±»Ńõ»Æ£¬Ņņ“Ė- NO2µÄ»¹ŌŌŚ-CH3µÄŃõ»ÆÖ®ŗ󣬼“·“Ó¦¢Ś£¬·“Ó¦¢ŪµÄĖ³Šņ²»Äܵߵ¹”£
ÓėHCl·“Ӧɜ³ÉŃĪ£¬²śĘ·²»“棬CµÄ²»±„ŗĶ¶ČĪŖ1£¬ĘäĶ¬·ÖŅģ¹¹ĢåæÉ·¢ÉśĖ®½ā·“Ó¦£¬Ōņŗ¬ėļü£¬ÓÖÓÉÓŚÖ»ŗ¬3ÖÖ²»Ķ¬»Æѧ»·¾³ĒāŌ×Ó£¬Ó¦øĆø߶ȶŌ³Ę£¬øł¾ŻÓŠŠņĖ¼Ī¬æÉŠ“³ö£ŗ(CH3)3CCONHCH3”¢(CH3)3CCH2CONH2”¢CH3CONHC£ØCH3£©3”¢HCON£ØCH3£©C£ØCH3£©3”¢CH3CON£ØCH2CH3£©2µČ”£- NH2ÓŠ»¹ŌŠŌ£¬Ņ×±»Ńõ»Æ£¬Ņņ“Ė- NO2µÄ»¹ŌŌŚ-CH3µÄŃõ»ÆÖ®ŗ󣬼“·“Ó¦¢Ś£¬·“Ó¦¢ŪµÄĖ³Šņ²»Äܵߵ¹”£

 ŌĶĮæģ³µĻµĮŠ“š°ø
ŌĶĮæģ³µĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĶʶĻĢā
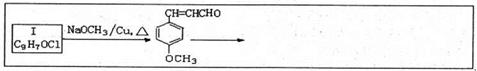
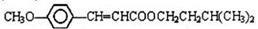 .ŗĻ³ÉĀ·ĻßČēĻĀ£ŗ
.ŗĻ³ÉĀ·ĻßČēĻĀ£ŗ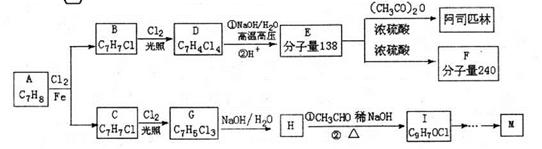
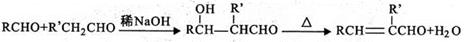
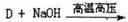 ____£¬E”śF____
____£¬E”śF____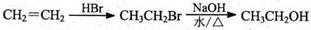
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĶʶĻĢā
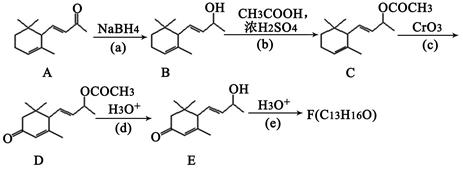
| A£®øĆĪļÖŹŹōÓŚ·¼Ļć×å»ÆŗĻĪļ | B£®øĆ·Ö×ÓÖŠÓŠ4ÖÖ²»Ķ¬ĄąŠĶµÄĒāŌ×Ó |
| C£®1moløĆĪļÖŹ×ī¶ąæÉĻūŗÄ2molNaOH | D£®øĆĪļÖŹÄÜ·¢ÉśŅų¾µ·“Ó¦ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
A£®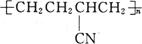 | B£® |
C£®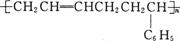 | D£®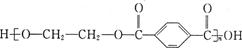 |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
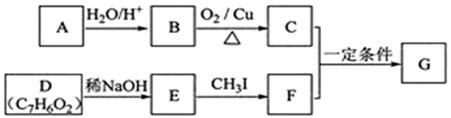

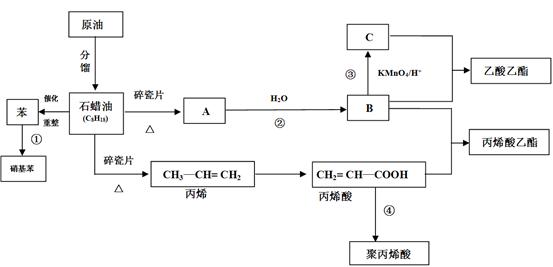
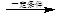 RCOCH = CHRØ@
RCOCH = CHRØ@²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĶʶĻĢā
 B ________________________________________________
B ________________________________________________ ±ūĻ©ĖįŅŅõ„ _______________________________
±ūĻ©ĖįŅŅõ„ _______________________________²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĢīæÕĢā
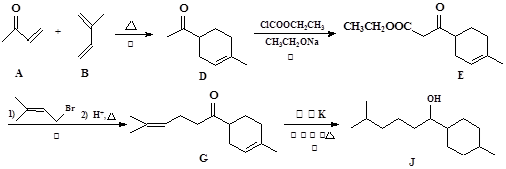
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗĶʶĻĢā
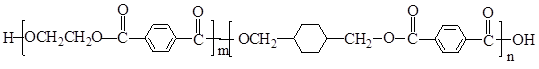
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ²»Ļź ĢāŠĶ£ŗµ„Ń”Ģā
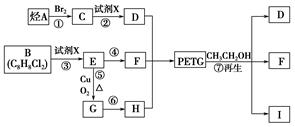
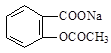 ×Ŗ±äĪŖ
×Ŗ±äĪŖ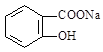 µÄ·½·ØŹĒ£ŗ
µÄ·½·ØŹĒ£ŗ| A£®Óė×ćĮæµÄNaOHČÜŅŗ¹²ČČŗó£¬ŌŁĶØČėCO2 | B£®ČÜŅŗ¼ÓČČ£¬ĶØČė×ćĮæµÄHCl |
| C£®ÓėĻ”H2SO4¹²ČČŗ󣬼ÓČė×ćĮæµÄNaHCO3 | D£®ÓėĻ”H2SO4¹²ČČŗ󣬼ÓČė×ćĮæµÄNaOH |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com