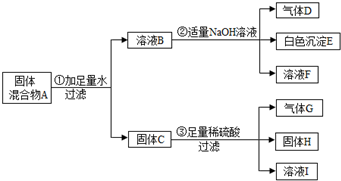
������A��B��C���־�����Ԫ�ص������������֮���ת����ϵ��ͼ�����ֲ�������ȥ����ʾ��
��1������д��Bת��ΪC���ܵ��������ӷ���ʽ
��
��
��A��C��Ӧ�����ӷ���ʽ
��
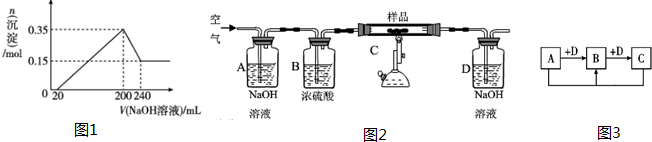
��2����������B����һϵ�з�Ӧ���Եõ�����E����һ��������Mg��E�Ļ����Ͷ��500mLϡ�����У�����ȫ���ܽⲢ�������壮����Ӧ��ȫ����������Һ�м���NaOH��Һ�����ɳ��������ʵ��������NaOH��Һ�������ϵ��ͼ�������������Mg����Ϊ
��NaOH��Һ���ʵ���Ũ��Ϊ
��
�������仯�����ڹ��á��ճ�������ռ�зdz���Ҫ�ĵ�λ���˽������仯��������ʺ���;�dz���Ҫ����ش��������⣺
��3��������Ȼ������
̬������롱���ϡ������ڣ����������г�������ɫ�����Ϳ�ϵ���
���ѧʽ��
��4���о��������ɴ�����������Ӧ�Ļ�ѧ����ʽ
��
��
��5�����ж�Fe
2+�ļ��鷽������������
��
A����ij��Һ�е���KSCN��Һ��Ѫ��ɫ��˵������Fe
2+B����ij��Һ��ͨ��Cl
2��Ȼ���ټ���KSCN��Һ���Ѫ��ɫ��˵��ԭ��Һ�к���Fe
2+C����ij��Һ�м�NaOH��Һ�ð�ɫ����������Ϊ���ɫ��˵������Һ�к���Fe
2+��6�����Ͻ����������ݣ�
| �� |
| ̼�ظ֣�Fe��C��Mn��Si�� | �Ͻ�� |
| ��̼�� | ��̼�� | ��̼�� | ̼�ظ�+Cr��Mn��W��Ni��Co�� |
| ��̼����0.3% | ��̼��0.3%��0.6% | ��̼����0.6% |
| ���Ժã�ǿ�ȵ� | ���Ժá�ǿ�Ⱥ� | Ӳ���� | ������������ |
ijͬѧ���ʵ��ͨ���ⶨ��������̼������������ȷ���ֵ����࣬ȡ15gij̼�ظ���Ʒ��������ʵ�����̽��в�����
��A��B��������
��
�ڳ�ּ�����ȫ��Ӧ��Dװ����������0.22g������Ʒ����
�֣�
��û��A��Bװ�ã�ʵ����
���ƫ�ߡ�����ƫ�͡���Ӱ�족����





 ij��̼���̿����Ҫ�ɷ���MnCO3��MnO2��FeCO3��MgO��SiO2��Al2O3�ȣ���֪̼����������ˮ��һ������������Ĥ��ⷨ���¼��������ڴ�̼���̿�����ȡ�����̣��������£�
ij��̼���̿����Ҫ�ɷ���MnCO3��MnO2��FeCO3��MgO��SiO2��Al2O3�ȣ���֪̼����������ˮ��һ������������Ĥ��ⷨ���¼��������ڴ�̼���̿�����ȡ�����̣��������£�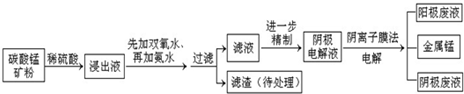

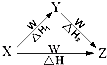 X��Y��Z��W��������֮������ͼ��ʾ��ת����ϵ����������δдȫ��������֪��Ӧ��֮��Ĺ�ϵ���ϵ�ʽ��H=��H1+��H2����X��Y�����Ǣ�C��CO ��AlCl3��Al��OH��3��Fe��Fe��NO3��2��Na2CO3��NaHCO3��������
X��Y��Z��W��������֮������ͼ��ʾ��ת����ϵ����������δдȫ��������֪��Ӧ��֮��Ĺ�ϵ���ϵ�ʽ��H=��H1+��H2����X��Y�����Ǣ�C��CO ��AlCl3��Al��OH��3��Fe��Fe��NO3��2��Na2CO3��NaHCO3��������