���к͵ζ��ķ����ⶨNaOH��Na
2CO
3�Ļ����Һ��NaOH�ĺ����������ڻ��Һ�м��������BaCl
2��Һ��ʹNa
2CO
3��ȫת���BaCO
3������Ȼ���ñ�����ζ�����֪�������ָʾ����ɫ��pH��Χ���ټ���3.1��4.4 �ڼ���4.4��6.2 �۷�̪8.2��10����
��1���ζ�ʱӦѡ��
��ָʾ����
��2���жϵ���ζ��յ��ʵ��������
��
��3�����в����ᵼ���ռ���Ʒ��NaOH�����ⶨֵƫ�ߵ���
A����ƿ������ˮϴ��δ�ô���Һ��ϴ
B����ʽ�ζ���������ˮϴ��δ�ñ�Һ��ϴ
C���ڵζ�ǰ�����ݣ��ζ���������ʧ
D���ζ�ǰƽ�Ӷ������ζ��������Ӷ���
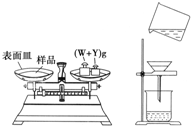
��4��Ϊ�ⶨij�ռ���Ʒ��NaOH�ĺ���������Ʒ������ΪNa
2CO
3����ijͬѧ��������ʵ�飺ȷ��ȡ5.0g��Ʒ���Ƴ�250mL��Һ��Ȼ������θ�ȡ���ƺõ��ռ���Һ20.00mL������������ˮϴ������ƿ�У��ֱ���������BaCl
2��Һ��������ƿ�и�����1��2��ָʾ������Ũ��Ϊ0.2000mol?L
-1�������Һ���еζ���������ݼ�¼�����
| ʵ���� | V���ռ���Һ��/mL | V��HCl��/mL |
| ������ | ĩ���� |
| 1 | 20.00 | 0.80 | 21.00 |
| 2 | 20.00 | 1.00 | 20.80 |
| 3 | 20.00 | 0.20 | 22.80 |
���ݱ������ݣ�������ռ���Ʒ�к�NaOH����������Ϊ
%����С���������λ���֣�
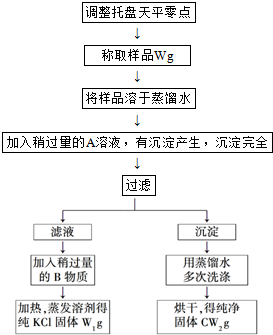
��5����ij��Ʒ������NaOH��Na
2CO
3��NaHCO
3�е�һ�ֻ�������ɣ�Ϊȷ������ɣ�ijͬѧ��������ʵ�飺
ȷ��ȡmg��Ʒ���Ƴ�250mL��Һ��ȡ���ƺõ���Һ20.00mL����ƿ�У�����2�η�̪��ָʾ������Ũ��Ϊcmol?L
-1�������Һ���еζ����յ㣬���������Һv
1ml��Ȼ���ٵμ�2�μ��ȼ�����Ũ��Ϊcmol?L
-1�������Һ���еζ����յ㣬���������Һv
2ml��v
1��v
2����Ϊ0����
����v
1��v
2��ֵ��С�ж���Ʒ����ɣ��û�ѧʽ��ʾ����
��v
1��v
2��v
1=v
2��v
1��v
2��



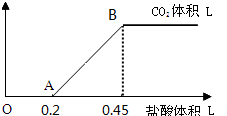
 ͨ��ѧ��Ĭд����ϵ�д�
ͨ��ѧ��Ĭд����ϵ�д� ���ƽ̸�������ѡ����ĩ���100��ϵ�д�
���ƽ̸�������ѡ����ĩ���100��ϵ�д�

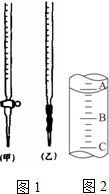 �Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����xֵ��ͨ���������ϸ�С��ͬѧͨ�������ѯ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O
�Ҷ����������ᣬij��ѧѧϰС���ͬѧ��̽���ⶨ���ᾧ�壨H2C2O4?xH2O����xֵ��ͨ���������ϸ�С��ͬѧͨ�������ѯ�ã�����������ˮ��ˮ��Һ����������KMnO4��Һ���еζ���2MnO4-+5H2C2O4+6H+=2Mn2++10CO2��+8H2O