
����Ŀ���Ǽ��㶹����һ�����Ƶ���ʯ��ҩ��ϳ�·������ͼ��ʾ��

��֪��
RCOOR'+R'OH![]() RCOOR'+ R'OH��R��R'��R'����������
RCOOR'+ R'OH��R��R'��R'����������
��1��A���ڷ���������ṹ��ʽ��______________________��B�������Ĺ�������________________��
��2��C��D�ķ�Ӧ������___________________��
��3��E����֬�ࡣ�����Ҵ�Ϊ�л�ԭ�ϣ�ѡ�ñ�Ҫ�����Լ��ϳ�E��д���йػ�ѧ����ʽ��______________________________��
��4����֪��2E![]() F+C2H5OH��F������������
F+C2H5OH��F������������![]() ��___________��
��___________��
��5����D��FΪԭ�Ϻϳ��Ǽ��㶹�ط�Ϊ������Ӧ��д���йػ�����Ľṹ��ʽ��

____________________________________
���𰸡� ![]() ���� ȡ����Ӧ 2C2H5OH+O2
���� ȡ����Ӧ 2C2H5OH+O2![]() 2CH3CHO+2H2O 2CH3CHO+O2
2CH3CHO+2H2O 2CH3CHO+O2![]() 2CH3COOH C2H5OH��CH3COOH
2CH3COOH C2H5OH��CH3COOH![]() CH3COOC2H5+H2O
CH3COOC2H5+H2O ![]()
![]()
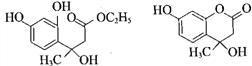
����������1��A�Ƿ�����������C�ķ���ʽ���Ƴ�A��Ӧ��6��̼ԭ�ӣ���AΪ�����ṹ��ʽΪ![]() ��A��B����ȡ����Ӧ�����룭NO2�����B�й���������������2������C��D�ķ���ʽ��C��D���������ǻ�ȡ��������λ�ã������ķ�Ӧ��ȡ����Ӧ����3��E��������һ�������������Ҵ���F����E���������������Ҵ���ȡ�������������ò����Ҵ�ͨ�������Ʊ����ᣬ�ٺ���һ�����Ҵ�ͨ��������Ӧ�õ����������������ķ�Ӧ��2CH3CH2OH+O2
��A��B����ȡ����Ӧ�����룭NO2�����B�й���������������2������C��D�ķ���ʽ��C��D���������ǻ�ȡ��������λ�ã������ķ�Ӧ��ȡ����Ӧ����3��E��������һ�������������Ҵ���F����E���������������Ҵ���ȡ�������������ò����Ҵ�ͨ�������Ʊ����ᣬ�ٺ���һ�����Ҵ�ͨ��������Ӧ�õ����������������ķ�Ӧ��2CH3CH2OH+O2 ![]() 2CH3CHO+2H2O��2CH3CHO+O2
2CH3CHO+2H2O��2CH3CHO+O2![]() 2CH3COOH��CH3CH2OH��CH3COOH
2CH3COOH��CH3CH2OH��CH3COOH![]() CH3COOCH2CH3��H2O����4�������֪ת��������ԭ���غ㣬�Ƴ�F�ķ���ʽΪC6H10O3��������Ϣ���Լ��Ǽ��㶹�صĽṹ��ʽ���Ƴ�F�Ľṹ��ʽΪCH3CH2OOCCH2COCH3��������
CH3COOCH2CH3��H2O����4�������֪ת��������ԭ���غ㣬�Ƴ�F�ķ���ʽΪC6H10O3��������Ϣ���Լ��Ǽ��㶹�صĽṹ��ʽ���Ƴ�F�Ľṹ��ʽΪCH3CH2OOCCH2COCH3��������![]() ��������
��������![]() ����5�������ǻ��㶹�صĽṹ��ʽ���Լ���2���ķ�����C��D�İ������ǻ��ֱ��ڱ����ļ�λ���ֱ�Ϊ
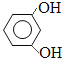
����5�������ǻ��㶹�صĽṹ��ʽ���Լ���2���ķ�����C��D�İ������ǻ��ֱ��ڱ����ļ�λ���ֱ�Ϊ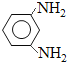 ��
�� ��F�Ľṹ��ʽΪCH3CH2OOCCH2COCH3��F��D������֪��һ����Ӧ�����м����1���м����1�Ľṹ��ʽΪ��
��F�Ľṹ��ʽΪCH3CH2OOCCH2COCH3��F��D������֪��һ����Ӧ�����м����1���м����1�Ľṹ��ʽΪ��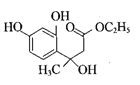 ���ٷ�����֪�ڵķ�Ӧ�����м����2��
���ٷ�����֪�ڵķ�Ӧ�����м����2��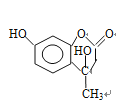 ��Ȼ������ȥ��Ӧ��ˮ�����Ǽ��㶹�ء�
��Ȼ������ȥ��Ӧ��ˮ�����Ǽ��㶹�ء�


 ��ʦ�㲦��ϵ�д�
��ʦ�㲦��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������˵��������ǣ���������
A. ��ͭ��ӡˢ��·��������ͭ�Ļ�ԭ��
B. �������ȷ�Ӧ�����Ӹֹ�
C. ��ë����˿��������Ҫ�ɷֶ�����ά��
D. ��������ͨ�ֶ�����̼�Ͻ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪��2Zn(s)+O2(g)![]() 2ZnO(s) ��H=701.0 kJ��mol1
2ZnO(s) ��H=701.0 kJ��mol1
2Hg(l)+O2(g)![]() 2HgO(s) ��H=181.6 kJ��mol1
2HgO(s) ��H=181.6 kJ��mol1
��ӦZn(s)+HgO(s)![]() ZnO(s)+Hg(l)�Ħ�HΪ
ZnO(s)+Hg(l)�Ħ�HΪ
A��+519.4 kJ��mol1 B��+259.7 kJ��mol1 C��259.7 kJ��mol1 D��519.4 kJ��mol1
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪KMnO4��Ũ���ᷴӦ�Ļ�ѧ����ʽΪ��2KMnO4+16HCl(Ũ)��2MnCl2+8H2O+2KCl+5Cl2�����ش��������⣺
��1���÷�Ӧ�����ӷ���ʽΪ________________________________________________��
��2����������HClռHCl������__________________________��
��3������״������11.2L��������ʱ���÷�Ӧת�Ƶĵ�����Ϊ________________����NAΪ�����ӵ�������ֵ����
��4��15.8 g KMnO4��100 mL 12 mol/LŨ������ȫ��Ӧ������HCl�ӷ������ڷ�Ӧ�����Һ�м���������AgNO3��Һ��������____________________g��������Ҫ��д��������̣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������������������![]() ��
��![]() ������ ������ ��
������ ������ ��![]() �����л�Ϊͬ���칹�����____________(�����)�������֮��Ĺ�ϵΪ_____________��
�����л�Ϊͬ���칹�����____________(�����)�������֮��Ĺ�ϵΪ_____________��
�ڢۢܢ��������ʰ����ǵķе��ɸߵ��͵�˳��������ȷ����_____________(�����)
��ij��A���л���ѧ��ҵ�Ļ���ԭ�ϣ������������������һ�����ҵ�ʯ�ͻ�����չˮƽ��A��һ�������¿ɷ�����ͼ��ʾ��ת������ش��������⣻

(1)д��A�ĵ���ʽ________��E�Ľṹ��ʽΪ______________
(2)д�����з�Ӧ��ѧ����ʽ����ע���ۢݷ�Ӧ����
�� _____________________________________________
�� _____________________ ����Ӧ����________;
�� _____________________ ����Ӧ����________;
(3)��ȥB�л��е���������A�����õ��Լ�Ϊ___________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ϩ��ʯ�ͻ�������Ҫԭ�ϣ�һ�������¿ɷ�������ת��
(1)A�Ľṹ��ʽΪ____________
(2)D�������Ҵ���Ӧ����E�Ļ�ѧ����ʽΪ_____________________________________________
(3)  ������NaOHˮ��Һ������Ӧ�Ļ�ѧ����ʽΪ_________________
������NaOHˮ��Һ������Ӧ�Ļ�ѧ����ʽΪ_________________
(4)B�ж���ͬ���칹����д�����м��ܷ���������Ӧ���ܷ���������Ӧ��2��ͬ���칹��Ľṹ��ʽ_________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����84����Һ������Чɱ�����H1N1�Ȳ�����ijͬѧ������һƿ����¶ʿ������84����Һ����������������Ϻ�����Һ��װ˵���õ�������Ϣ����84����Һ������25%NaClO��1 000 mL���ܶ�1.19 g��cm��3��ϡ��100��(�����)��ʹ�á�ͬѧ��������¶ʿ������84����Һ�����䷽������NaClO��������480 mL��25% NaClO������Һ������˵����ȷ���� ( )
A. ����ʱ��Ҫ�IJ����������ձ�����Ͳ������ƿ���һ�ֲ�������
B. ����ƿ������ˮϴ����Ӧ��ɲ���������Һ������
C. ����ʱˮ�Ӷ���Ӧ������ֽ������
D. ��Ҫ������NaClO��������Ϊ149 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����֪A��B��C��D��E��FΪԪ�����ڱ���ǰ��������ԭ�������������������Ԫ��.����A��B��C��D�˵����֮��Ϊ36��A��Cԭ�ӵ�����������֮�͵���Bԭ�� �Ĵ�����������Dԭ��������ΪBԭ����������������EԪ�����������Ϊ������F�ļ۵�������C�ĺ˵������ȡ�
��1�����й�����������Ԫ�ص�˵����ȷ����_________��
a.B��C��D��ԭ�Ӱ뾶�ɴ�С��˳��Ϊ��D >C >B
b.E��F���������������
c.A��B��C��D����Ԫ���е縺�Ժ͵�һ���������ľ�ΪB
d.B��C�γɵĻ������п��ܺ��зǼ��Լ�
e.A��C��F��λ�����ڱ���3��
��2��B����������ͬ��������.������ˮ���ܽ�Ƚϴ����_______���ѧʽ)��
��3��EA2��A2B�۵�ϸߵ��� _______(�ѧʽ����ԭ����_________��
��4��D��B�����γ����ַ��ӣ�����DB2��������ԭ�ӵ��ӻ�������____�����з��ӻ���������DB3�ṹ���Ƶ���____________��
a. NH3 b. SO32- c.NO3- d.PCl3
��5����֪B��F���γ����ֻ�����侧������ͼ��ʾ.�����ʱ����ת��Ϊ�ҵ�ԭ��Ϊ ___________�����Ҿ����ܶ�Ϊpg/cm3�����Ҿ����ľ����߳�a =________nm(�ú�P��NA��ʽ�ӱ�ʾ��

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ij��̬����������CnHm��һ����������H2�ӳ�ΪCnHm+x��ȡCnHm��H2������干60mL����ʵ�飬������������H2��ռ����ı仯����Ӧ��õ��������������Ҳ��ͬ����Ӧǰ���������H2��ռ�����V(H2)�ͷ�Ӧ�����������V(��Ӧ����)�Ĺ�ϵ��ͼ��ʾ(�����������ͬ��ͬѹ�²ⶨ)���ɴ˿�֪x����ֵΪ
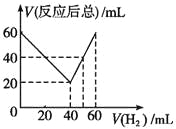
A. 4 B. 3 C. 2 D. 1
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com