
�� 12�� ��A~I�ֱ��ʾ��ѧ��ѧ�г�����һ���� �ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�
�ʣ�����A��IΪ��������������֮������ϵ��ͼ��ʾ�����ַ�Ӧ�������û���г���������֪GΪ����Ԫ�صĹ�̬�����A��B��C��D��E��F���������о���ͬһ��Ԫ�ء�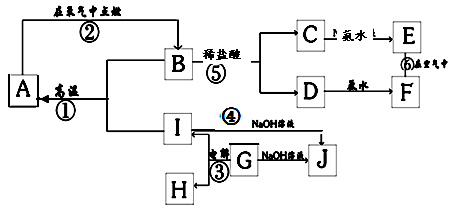
����д���пհף�
��1��A��B��C��D��E��F����������������ͬһ��Ԫ����Ԫ�����ڱ��е�λ���� ��
��2������C��Һ�������ӵķ����ǣ�д�������������ۣ�
��
��3��д�����з�Ӧ�Ļ�ѧ����ʽ�����ӷ���ʽ��
��Ӧ�ٵĻ�ѧ����ʽ�� ��
��Ӧ�ܵ����ӷ���ʽ�� ��
��Ӧ�Ļ�ѧ����ʽ�� ��
��4���������仯�ĽǶȿ�����Ӧ�٢ڢ��У����ڡ�H��0�ķ�Ӧ�� ������ţ���

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012���Ϻ����ɽ���������ѧ����ĩ��1�£����Ի�ѧ�Ծ����������� ���ͣ������
�����12�֣�
A��ʯ�ͻ�ѧ��ҵ����Ҫ�Ļ���ԭ�ϣ���AΪ�л�ԭ�ϣ����Լ���ѡ��������;���ϳ�һ�ֳ�����ҽ�ý����ṹ��ʽΪ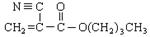 ��
��
��֪����ȩ������һ�������¿��Է������·�Ӧ��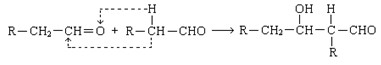
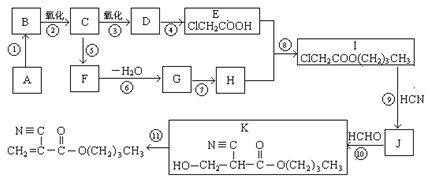
��������ش�
1��A�Ľṹ��ʽ��_________________��D�Ĺ����������� ��
2���ķ�Ӧ������ ��I��J����һ�������� ��
3������ȡ����Ӧ���У��Ӧ��ţ�____________����ķ�Ӧ������ ��
4��д���ݵĻ�ѧ����ʽ��_____________________________________________________��
5��F�ж���ͬ���칹�壬�����ܹ���NaOH��Һ�з�����Ӧ��ͬ���칹���� �֡�
6�������FΪԭ�Ͼ��������ϳ�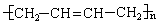 ����Ӧ�ķ�Ӧ���ͷֱ�Ϊ
����Ӧ�ķ�Ӧ���ͷֱ�Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ϻ����ɽ���������ѧ����ĩ��1�£����Ի�ѧ�Ծ��������棩 ���ͣ������
�����12�֣�
A��ʯ�ͻ�ѧ��ҵ����Ҫ�Ļ���ԭ�ϣ���AΪ�л�ԭ�ϣ����Լ���ѡ��������;���ϳ�һ�ֳ�����ҽ�ý����ṹ��ʽΪ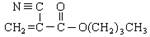 ��
��
��֪����ȩ������һ�������¿��Է������·�Ӧ��
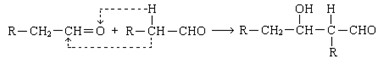
��������ش�
1��A�Ľṹ��ʽ��_________________��D�Ĺ����������� ��
2���ķ�Ӧ������ ��I��J����һ�������� ��
3������ȡ����Ӧ���У��Ӧ��ţ�____________����ķ�Ӧ������ ��
4��д���ݵĻ�ѧ����ʽ��_____________________________________________________��
5��F�ж���ͬ���칹�壬�����ܹ���NaOH��Һ�з�����Ӧ��ͬ���칹���� �֡�
6�������FΪԭ�Ͼ��������ϳ�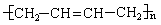 ����Ӧ�ķ�Ӧ���ͷֱ�Ϊ
����Ӧ�ķ�Ӧ���ͷֱ�Ϊ
��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012���������ϴ�ѧ���и�һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ������
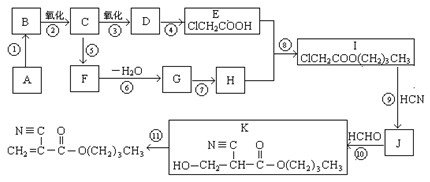
(12��)������A��һ����Ҫ�Ĺ⻯ѧ�Լ���A��H֮���ת����ϵ����ͼ��ʾ�������ַ�Ӧ�������û���г���

��֪��������A�к����������Ӻ�һ�ִ�������λ����ɵ������ӣ��������ӵĸ�����Ϊ3��1��3���������и�Ԫ�ص�����������C��ͬ����Է���������C��2����C��D��Ϊ�����Һ���Ԫ��������ͬ��C��ʹ����ʯ��ˮ����ǣ�E��һ�ֺ���ɫ�����ϣ�F����ɫ��Ӧ����ɫ��I��ʹKSCN��Һ�Ժ�ɫ��
�ش��������⣺
(1)C�ĵ���ʽΪ�� ��A�Ļ�ѧʽΪ�� ��
(2)D+E��C+G�Ļ�ѧ����ʽ�� ��
(3)H+H2O2+HCl��I�����ӷ���ʽ�� ��
(4)��A����Һ�е������Ը��������Һ�����������Һ��ɫ��ȥ������A����Һ�е������軯����Һ����Һ�ʺ�ɫ�����������������Ҫԭ����
_________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ�갲��ʡ���������꼶��һ�ν�ѧ������鿼�Ի�ѧ�Ծ��������棩 ���ͣ������
(12��)����A��B��C��D��E���ֶ�����Ԫ�أ���ԭ��������������A��C�Ļ�̬ԭ�ӵ����Ų���δ�ɶԵ�����������������ȣ���A�������������Ǵ�����������2����D�������ӡ�C�������Ӿ�����ԭ�ӵĵ��Ӳ�ṹ��ͬ����D�ĵ�����C�ĵ��ʷ�Ӧ������D2C��D2C2���ֻ����E����������������������������l��
(1)2011���ǹ��ʻ�ѧ�꣬����һ����Ҫ��Ǽ��������˻�ŵ������ѧ��100���ꡣ��������˷���I����(Ra)����(Po)Ԫ�أ�����E����ͬ���壬��λ�� �塣
(2)E�Ļ�̬ԭ�ӵĺ�������Ų�ʽ�� ��A��B��C����Ԫ���е�һ������������ ��B��c��D��ԭ�Ӱ뾶��С�����˳��Ϊ (����Ԫ�ط��� ��ʾ)��
(3)D2C2��AC2��Ӧ�Ļ�ѧ����ʽΪ ����д���÷�Ӧ��һ���� ��
(4)B����Ԫ���γ������̬�⻯��Ŀռ乹��Ϊ �����й��ۼ�����Ϊ (��Լ���Ǽ��Լ�)����֪B��B����Ϊ946 kJ��mol-1��H-H����Ϊ
436 kJ��mol-1��B��H����Ϊ391 kJ��mol-1����B2��H2��Ӧ���Ȼ�ѧ����ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com