
��������Ҫ������Դ��������Ϊ�������õĹؼ��������ǵ�ǰ��ע���ȵ�֮һ��
(1)���������ȼ�ϣ���ȼ�ղ���Ϊ________��
(2)NaBH4��һ����Ҫ�Ĵ������壬����ˮ��Ӧ�õ�NaBO2���ҷ�Ӧǰ��B�Ļ��ϼ۲��䣬�÷�Ӧ�Ļ�ѧ����ʽΪ__________________________________________����Ӧ����1 mol NaBH4ʱת�Ƶĵ�����ĿΪ________��
 (3)����ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺
(3)����ɽ����л�������û�����ͱ�֮��Ŀ��淴Ӧ��ʵ������ͼ��⣺
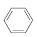 (g)
(g) 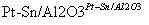 (g)��3H2(g)��
(g)��3H2(g)��
��ij�¶��£�������ܱ������м��뻷���飬����ʼŨ��Ϊa mol��L��1��ƽ��ʱ����Ũ��Ϊb mol��L��1���÷�Ӧ��ƽ�ⳣ��K��________��
(4)һ�������£���ͼ��ʾװ�ÿ�ʵ���л���ĵ绯ѧ����(���������л���)��
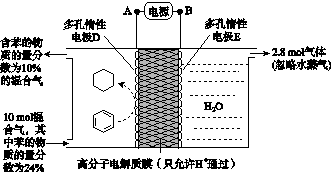
�ٵ����е����ƶ�����Ϊ________��(��A��D��ʾ)
������Ŀ�����ĵ缫��ӦʽΪ__________________��
�۸ô���װ�õĵ���Ч�ʦǣ�____________________��(�ǣ�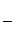 ��100%������������С�����1λ)
��100%������������С�����1λ)
[��]��(1)H2O
(2)NaBH4��2H2O===NaBO2��4H2����
4NA��2.408��1024
(3)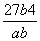 mol3��L��3
mol3��L��3
(4)��A��D����C6H6��6H����6e��===C6H12����64.3%[����] (2)BԪ�ط�Ӧǰ�ϼ۲��䣬������Ӧ��������NaBO2��֪����ӦǰNaBH4��B�Ļ��ϼ�Ҳ�ǣ�3�ۣ���HΪ��1�ۣ�ˮ����Ԫ�ػ��ϼ�Ϊ��1�ۣ��������з�Ӧ����������������Ԫ�ػ��ϼۿ�ȷ��ʧȥ��������(3)���ݻ�ѧ����ʽ��֪������H2Ϊ3b mol/L�����Ļ�����b mol/L����ƽ��ʱ������Ϊ(a��b) mol/L��������ʽȷ��ƽ�ⳣ����(4)�ٱ����ɻ������ǵ��ⷴӦ��Ϊ��ԭ��Ӧ�����缫D���������缫E�������������е����ƶ�����A��D����Ŀ������ǻ����飬������ͨ�����ӽ���Ĥ�������ƶ�����缫��ӦʽΪC6H6��6H����6e��===C6H12������������2.8 mol���壬��������OH���������ŵ������������ת�Ƶ���11.2 mol�����������ı������ʵ�����x mol��ͬʱ����x mol�����飬���缫��Ӧʽ��֪ת�Ƶĵ���Ϊ6x mol������ݵ����غ�֪��ͬʱ����������(11.2 mol��6x mol)��2��5.6 mol��3x mol�����ݻ������ɷֿ���ʽ 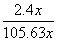 ��10%�����x��1.2����˴���װ�õĵ���Ч��Ϊ
��10%�����x��1.2����˴���װ�õĵ���Ч��Ϊ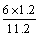 ��64.3%��
��64.3%��


 С��ſ�ʱ��ҵϵ�д�
С��ſ�ʱ��ҵϵ�д� һ������ϵ�д�
һ������ϵ�д� �Ƹ�С״Ԫ���ֳ������ϵ�д�
�Ƹ�С״Ԫ���ֳ������ϵ�д� �¸��̵�ѧϵ�д�
�¸��̵�ѧϵ�д� ����ͬѧһ����ʦȫ�źþ�ϵ�д�
����ͬѧһ����ʦȫ�źþ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ijС����CoCl2��6H2O��NH4Cl��H2O2��Ũ��ˮΪԭ�ϣ��ڻ���̿���£��ϳ��˳Ȼ�ɫ����X��Ϊȷ������ɣ���������ʵ�� ��
�ٰ��IJⶨ����ȷ��ȡw g X��������ˮ�ܽ⣬ע����ͼ��ʾ������ƿ�У�Ȼ����μ�������10%NaOH��Һ��ͨ��ˮ����������ƷҺ�еİ�ȫ����������V1 mL cl mol��L��1���������Һ���ա�����������ȡ�½���ƿ����c2 mol��L��1NaOH����Һ�ζ���ʣ��HCl�����յ�ʱ����V2 mL NaOH��Һ��
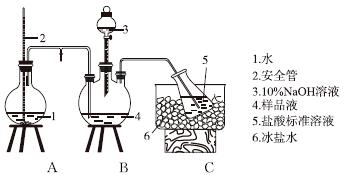
���IJⶨװ��(��ʡ�Լ��Ⱥͼг�װ��)
���ȵIJⶨ��ȷ��ȡ��ƷX�������Һ����AgNO3����Һ�ζ���K2CrO4��ҺΪָʾ���������ֵ���ɫ����������ʧΪ�յ�(Ag2CrO4Ϊש��ɫ)��
�ش��������⣺
(1)װ���а�ȫ�ܵ�����ԭ����__________________________________________��
(2)��NaOH����Һ�ζ���ʣ��HClʱ��Ӧʹ��________ʽ�ζ��ܣ���ʹ�õ�ָʾ��Ϊ________��
(3)��Ʒ�а���������������ʽΪ________��
(4)�ⶨ��ǰӦ�ö�װ�ý��������Լ��飬�������Բ��òⶨ�����________(�ƫ�ߡ���ƫ�͡�)��
(5)�ⶨ�ȵĹ����У�ʹ����ɫ�ζ��ܵ�ԭ����____________________���ζ��յ�ʱ������Һ��c(Ag��)��2.0��10��5 mol��L��1��c(CrO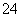 )Ϊ________mol��L��1��[��֪��Ksp(Ag2CrO4)��1.12��10��12]
)Ϊ________mol��L��1��[��֪��Ksp(Ag2CrO4)��1.12��10��12]
(6)���ⶨ����ƷX���ܡ������ȵ����ʵ���֮��Ϊ1��6��3���ܵĻ��ϼ�Ϊ________���Ʊ�X�Ļ�ѧ����ʽΪ______________________________________��X���Ʊ��������¶Ȳ��ܹ��ߵ�ԭ����________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£�10 mL 0.40 mol/L H2O2��Һ�������ֽ⡣��ͬʱ�̲������O2�����(������Ϊ��״��)���±���
| t/min | 0 | 2 | 4 | 6 | 8 | 10 |
| V(O2)/mL | 0.0 | 9.9 | 17.2 | 22.4 | 26.5 | 29.9 |
������������ȷ����(��Һ����仯���Բ���)(����)
A��0��6 min��ƽ����Ӧ���ʣ�
v(H2O2)��3.3��10��2mol��L��1��min��1
B��0��6 min��ƽ����Ӧ���ʣ�
v(H2O2)<3.3��10��2mol��L��1��min��1
C����Ӧ��6 minʱ��c(H2O2)��0.30 mol/L
D����Ӧ��6 minʱ��H2O2�ֽ���50%
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����������������OH�������·���ˮ�ⷴӦ��
O2NC6H4COOC2H5��OH�� O2NC6H4COO����C2H5OH
O2NC6H4COO����C2H5OH
���ַ�Ӧ��ij�ʼŨ�Ⱦ�Ϊ0.050 mol��L��1��15 ��ʱ���O2NC6H4COOC2H5��ת��������ʱ��仯�����������ʾ���ش��������⣺
| t/s | 0 | 120 | 180 | 240 | 330 | 530 | 600 | 700 | 800 |
| ��/% | 0 | 33.0 | 41.8 | 48.8 | 58.0 | 69.0 | 70.4 | 71.0 | 71.0 |
(1)��ʽ����÷�Ӧ��120��180 s��180��240 s �����ƽ����Ӧ����________��________���Ƚ����ߴ�С�ɵó��Ľ�����____________________��
(2)��ʽ����15 ��ʱ�÷�Ӧ��ƽ�ⳣ��________��
(3)Ϊ���O2NC6H4COOC2H5��ƽ��ת���ʣ������ʵ����Ʒ�Ӧ�¶��⣬���ɲ�ȡ�Ĵ�ʩ��________(Ҫ��д������)��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
H2O2��һ����ɫ������ԭ�Լ����ڻ�ѧ�о���Ӧ�ù㷺��
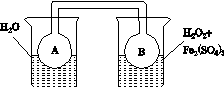
(1)ijС������ͬŨ��Fe3���Ĵ��£�̽��H2O2Ũ�ȶ�H2O2�ֽⷴӦ���ʵ�Ӱ�졣��ѡ�Լ���������30%H2O2��Һ��0.1 mol��L��1Fe2(SO4)3��Һ������ˮ����ƿ��˫������ˮ�ۡ����ܡ��������ܡ���Ͳ�����������ˮԡ�ۡ�ע������
��д����ʵ��H2O2�ֽⷴӦ����ʽ����������ת�Ƶķ������Ŀ��______________________________��
�����ʵ�鷽�����ڲ�ͬH2O2Ũ���£��ⶨ________(Ҫ������õ�������ֱ�����ַ�Ӧ���ʴ�С)��
�����ʵ��װ�ã����ͼ�е�װ��ʾ��ͼ��
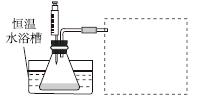
�ܲ����±���ʽ���ⶨʵ�������������ʵ�鷽��(�г���ѡ�Լ���������¼�Ĵ��������������ⶨ�����ݣ���������ĸ��ʾ)��
| ���������� ʵ����� ���� | V[0.1 mol��L��1 Fe2(SO4)3]/mL | ���� | |
| 1 | a | ���� | |
| 2 | a | ���� |
(2)����ͼ(a)��(b)�е���Ϣ����ͼ(c)װ��(��ͨ��A��Bƿ���ѳ���NO2����)����ʵ�顣�ɹ۲쵽Bƿ��������ɫ��Aƿ�е�__________(����dz��)����ԭ����____________________________��
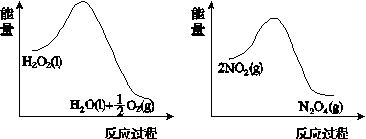
����������(a)������������������������(b)
(c)
ͼ21
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�����仯�����������������ϵ���С�
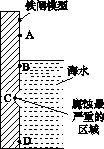
(1)��ͼ��ʵ�����о���ˮ����բ��ͬ��λ��ʴ���������ʾ��ͼ��
�ٸõ绯��ʴ��Ϊ________��
��ͼ��A��B��C��D�ĸ�������������������________(����ĸ)��
(2)�÷���Ƥ��ȡ����(Fe2O3)�IJ�������ʾ��ͼ���£�
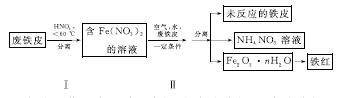
�ٲ�������¶ȹ��ߣ�����������ֽ⡣����ֽ�Ļ�ѧ����ʽΪ______________________________��
�ڲ�����з�����Ӧ��4Fe(NO3)2��O2��(2n��4)H2O===2Fe2O3��nH2O��8HNO3����Ӧ������HNO3�ֽ�����Ƥ�е���ת��ΪFe(NO3)2���÷�Ӧ�Ļ�ѧ����ʽΪ____________________________��
���������������У������֡���ɫ��ѧ��˼�����______(��дһ��)��
(3)��֪t ��ʱ����ӦFeO(s)��CO(g)Fe(s)��CO2(g)��ƽ�ⳣ��K��0.25��
��t ��ʱ����Ӧ�ﵽƽ��ʱn(CO)��n(CO2)��________��
������1 L�ܱ������м���0.02 mol FeO(s)����ͨ��x mol CO, t ��ʱ��Ӧ�ﵽƽ�⡣��ʱFeO(s)ת����Ϊ50%����x��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
һ���¶��£������������Ϊ1.0 L�ĺ����ܱ������з�����Ӧ��(����)
2CH3OH(g)  CH3OCH3(g)��H2O(g)
CH3OCH3(g)��H2O(g)
| ���� ��� | �¶�(��) | |||
| ��ʼ���ʵ���(mol) | ƽ�����ʵ���(mol) | |||
| CH3OH(g) | CH3OCH3(g) | H2O(g) | ||
| �� | 387 | 0.20 | 0.080 | 0.080 |
| �� | 387 | 0.40 | ||
| �� | 207 | 0.20 | 0.090 | 0.090 |
����˵����ȷ����(����)
A���÷�Ӧ������ӦΪ���ȷ�Ӧ
B���ﵽƽ��ʱ���������е�CH3OH����������������е�С
C���������з�Ӧ����ƽ������ʱ����������еij�
D������ʼʱ���������г���CH3OH 0.15 mol��CH3OCH3 0.15 mol��H2O 0.10 mol����Ӧ��������Ӧ�������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
���ݻ�Ϊ1.00 L�������У�ͨ��һ������N2O4��������ӦN2O4(g)2NO2(g)�����¶����ߣ�����������ɫ���
�ش��������⣺
(1)��Ӧ�Ħ�H________0(����ڡ���С�ڡ�)��100 ��ʱ����ϵ�и�����Ũ����ʱ��仯��ͼ��ʾ����0��60 sʱ�Σ���Ӧ����v(N2O4)Ϊ________mol��L��1��s��1����Ӧ��ƽ�ⳣ��K1Ϊ________��
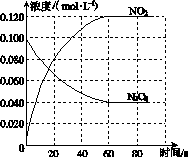
(2)100 ��ʱ��ƽ��ı䷴Ӧ�¶�ΪT��c(N2O4)��0.002 0 mol��L��1��s��1��ƽ�����ʽ��ͣ���10 s�ִﵽƽ�⡣
��T________100 ��(����ڡ���С�ڡ�)���ж�������____________________________��
����ʽ�����¶�Tʱ��Ӧ��ƽ�ⳣ��K2��_______________________________________
________________________________________________________________________��
(3)�¶�Tʱ��Ӧ��ƽ�����Ӧ�������ݻ�����һ�룬ƽ����________(�����Ӧ�����淴Ӧ��)�����ƶ����ж�������__________________________________________________
________________________________________________________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ʯī�ڲ�����������ҪӦ�á�ij����ʯī�к�SiO2(7.8%)��Al2O3(5.1%)��Fe2O3(3.1%)��MgO(0.5%)�����ʡ���Ƶ��ᴿ���ۺ����ù������£�
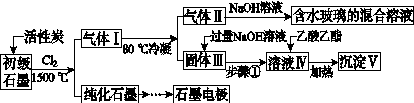
(ע��SiCl4�ķе�Ϊ57.6 �棬�����Ȼ���ķе������150 ��)
(1)��Ӧ����ͨ��Cl2ǰ����ͨһ��ʱ��N2����ҪĿ����____________________��
(2)���·�Ӧ��ʯī�����������ʾ�ת��Ϊ��Ӧ���Ȼ��������е�̼��������ҪΪ________�����������ij��õ�ˮ�����Ļ�ѧ��Ӧ����ʽΪ____________________________________________��
(3)�����Ϊ�����衢________��������Һ���е���������________��
(4)����Һ�����ɳ��������ܷ�Ӧ�����ӷ���ʽΪ______________________________________________��100 kg����ʯī�����ܻ�â�������Ϊ______kg��
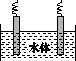
(5)ʯī��������Ȼˮ����ͭ���ĵ绯ѧ�����������ͼ����ʾ��ͼ��������Ӧ��ע��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com