
���ݻ���ͬ���ܱ������У��ֱ��������ĵ������������ڲ�ͬ�¶��·�����Ӧ��N2(g)��3H2(g)
 2NH3(g)�����ֱ��ڲ�ͬ��ʱ���ڲⶨ����NH3����������(y������ʾ��)�����ͼ����ͼ��ʾ����ش�
2NH3(g)�����ֱ��ڲ�ͬ��ʱ���ڲⶨ����NH3����������(y������ʾ��)�����ͼ����ͼ��ʾ����ش�
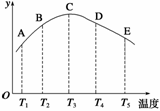
(1)A��B��C��D��E����У��϶�δ��ƽ��ĵ���
________________________________________________________________________��
(2)�˿��淴Ӧ������Ӧ��__________�ȷ�Ӧ��
(3)AC���������������ߣ�CE�����Ǽ��������ߣ��Դӻ�ѧ��Ӧ���ʺͻ�ѧƽ��ĽǶȷ�������˵������
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________
________________________________________________________________________��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����ʽΪC7H8��ij�л������ʹ���Ը��������Һ��ɫ������������ˮ��Ӧ����һ����������H2��ȫ�ӳɣ��ӳɺ���һ�ȴ����ͬ���칹����(����)��
A��3�� B��4��
C��5�� D��6��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��֪��Na2S2O3��H2SO4===Na2SO4��SO2����S����H2O�����и���ʵ���У���Ӧ����������(����)
| ��� | ��Ӧ�¶�/�� | Na2S2O3 | H2SO4 | H2O | ||
| ���/ mL | Ũ��/ mol��L��1 | ���/ mL | Ũ��/ mol��L��1 | ���/ mL | ||
| A | 10 | 5 | 0.2 | 5 | 0.1 | 10 |
| B | 10 | 5 | 0.1 | 5 | 0.1 | 10 |
| C | 30 | 5 | 0.1 | 5 | 0.1 | 10 |
| D | 30 | 5 | 0.2 | 5 | 0.2 | 10 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
ij�¶��£������Ϊ2 L���ܱ������г���1 mol A��b mol B���壬�������·�Ӧ��A(g)��B(g)
 2C(g)��5 min��Ӧ��ƽ��ʱn(A)Ϊ0.4 mol���ڷ�Ӧ��������ϵ���¶ȳ������ߣ�ʵ���û��������C�ĺ������¶ȵĹ�ϵ����ͼ��ʾ������������ȷ����(����)
2C(g)��5 min��Ӧ��ƽ��ʱn(A)Ϊ0.4 mol���ڷ�Ӧ��������ϵ���¶ȳ������ߣ�ʵ���û��������C�ĺ������¶ȵĹ�ϵ����ͼ��ʾ������������ȷ����(����)
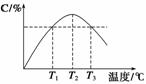
A��0��5 min��C���ʵ�ƽ����Ӧ����Ϊ0.04 mol��L��1��min��1
B��ͼ���¶�T1ʱ������Ӧ���ʵ����¶�T3ʱ������Ӧ����
C���÷�Ӧ�¶�T2ʱ��ƽ�ⳣ�������¶�T3ʱ��ƽ�ⳣ��
D��ͼ��T2ʱ����ֻ����ѹǿ���������淴Ӧ���ʲ��ı�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��N2(g)��3H2(g)
 2NH3(g)����H<0��ƽ����ϵ�У��������NH3ʱ������˵����ȷ����(����)
2NH3(g)����H<0��ƽ����ϵ�У��������NH3ʱ������˵����ȷ����(����)
A.�ı����������ʡ�ʱ��ͼ������ͼ��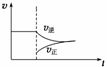
B���˹�����Q>K
C��ƽ����ϵ��NH3�ĺ�������
D��N2��ת��������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C�������أ�A����ʢ��CuCl2��Һ����ͭƬ��������B��C�����ھ�ʢ��AgNO3��Һ������˿����������B������˿��������0.108 g��C������˿��������0.216 gʱ��A����ͭƬ��������(����)
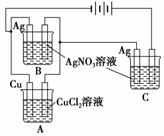
A��0.216 g�������������� B��0.108 g
C��0.064 g D��0.032 g
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
A��B��C����ǿ����ʣ�������ˮ�е�������������±���ʾ��
| ������ | Na����K����Cu2�� |
| ������ | SO |
��ͼ1��ʾװ���У��ס��ҡ��������ձ�������ʢ��������A��Һ��������B��Һ��������C��Һ���缫��Ϊʯī�缫����ͨ��Դ������һ��ʱ��������c�缫����������16 g�������¸��ձ�����Һ��pH����ʱ��t�Ĺ�ϵ��ͼ2��ʾ����ش��������⣺
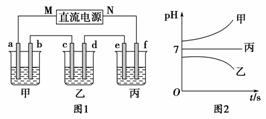
(1)MΪֱ����Դ��________����b�缫�Ϸ����ĵ缫��ӦΪ__________________________________________________________��
(2)����e�缫�����ɵ������ڱ�״���µ����Ϊ________��
(3)д�����ձ��е��ܷ�Ӧ�����ӷ���ʽ��__________________________________________________________��
(4)Ҫʹ���ձ��е�C��Һ�ָ���ԭ����״̬����Ҫ���еIJ�����(д��Ҫ��������ʺ�����)____________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�ҹ��зḻ���Ӣʯ��Դ������Ҫ�ɷ���ZrSiO4��������Al2O3��SiO2��Fe2O3�����ʣ�����ﯵ�����֮һ���£�
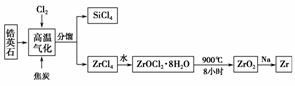
��֪���(Zr)�Ͻ��Ǻ˷�Ӧ��ȼ�ϰ��İ������ϣ��������(ZrO2)�����������������մɡ�
(1)���������и��������ķ�Ӧ��������ԭ��Ӧ(̼ת����CO)��������������________��ת��1.204��1024������ʱ������SiCl4________g��
(2)д��ZrCl4��ˮ��Ӧ�Ļ�ѧ����ʽ��______________________��д��ZrOCl2��8H2O��900�������·ֽ�Ļ�ѧ����ʽ��____________________________________��
3���й��ڶ�����������մɺ�ﯺϽ��˵������ȷ���ǡ���������
A.1 nm��10��10 m
B.ﯺϽ��Ӳ�ȱȴ��Ҫ��,C.������������մ����������ǽ�������
4һ������ȼ�ϵ�أ�һ��ͨ���������һ��ͨ�붡�飻������Dz���������Y2O3�������ZrO2���壬������״̬���ܴ���O2���������ڵ�����У�O2��������������������ƶ�����صĸ�����ӦʽΪ������������������������������������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������Һ�и�����Ũ�ȹ�ϵ��ȷ����(˫ѡ)(����)
A����pH�İ�ˮ��KOH��Һ��Ba(OH)2��Һ�У�c(NH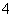 )��c(K��)��2c(Ba2��)
)��c(K��)��2c(Ba2��)
B����10 mL 0.1 mol·L��1 Na2CO3��Һ��ε���10 mL 0.1 mol·L��1�����У�c(Na��)>
c(Cl��)>c(HCO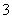 )>c(CO
)>c(CO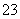 )
)
C����NH4HCO3��Һ�еμ�NaOH��Һ��pH��7��c(NH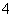 )��c(Na��)��c(HCO
)��c(Na��)��c(HCO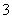 )��c(CO
)��c(CO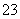 )
)
D��0.2 mol·L��1ijһԪ����HA��Һ��0.1 mol·L��1 NaOH��Һ�������Ϻ����Һ��2c(OH��)��c(A��)��2c(H��)��c(HA)
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com