
��2L����ѹ�Ƶĺ����ܱ�������ͨ��2molX��g����1molY��g����������Ӧ��2X��g��+Y��g��![]() 2Z��g�� ��H��0����ѹǿp��t����ͼ����ͼ�ף�ͼ��p2=0.85p1������ʱ�¶�����ʼ�¶���ͬ����ش��������⣺
2Z��g�� ��H��0����ѹǿp��t����ͼ����ͼ�ף�ͼ��p2=0.85p1������ʱ�¶�����ʼ�¶���ͬ����ش��������⣺
![]()
![]() ��1����ƽ��ʱZ�����ʵ���Ϊa����a=___________�����ӿ�ʼ��Ӧ��ƽ�⣬Y��ƽ������Ϊb����b=____________��
��1����ƽ��ʱZ�����ʵ���Ϊa����a=___________�����ӿ�ʼ��Ӧ��ƽ�⣬Y��ƽ������Ϊb����b=____________��
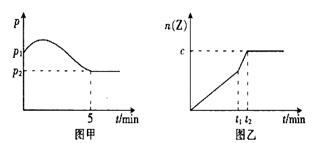
![]() ��2����ͬ�����½��з�Ӧ���ڴﵽƽ��ǰ��t1���ı�����һ��������������ͼ����ͼ�ҡ�
��2����ͬ�����½��з�Ӧ���ڴﵽƽ��ǰ��t1���ı�����һ��������������ͼ����ͼ�ҡ�
![]() A������ B������ C����ѹ D����ѹ E���Ӵ���
A������ B������ C����ѹ D����ѹ E���Ӵ���
![]() ����c=a����������_________�����ţ���ͬ����t2_______(���������������=����ͬ��5min
����c=a����������_________�����ţ���ͬ����t2_______(���������������=����ͬ��5min
![]() ����c��a����������__________��Y��ƽ������_________b
����c��a����������__________��Y��ƽ������_________b
![]() ����c��a����������___________��ƽ��ʱѹǿ__________p2
����c��a����������___________��ƽ��ʱѹǿ__________p2
![]()
![]()

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ�긣��ʡ�ĵ���У�߶��������¿���ѧ�Ծ����������� ���ͣ������
��14�֣���2L����ѹ�Ƶĺ����ܱ�������ͨ��2molX��g����1molY��g����������Ӧ��
2X��g��+Y��g�� 2Z��g������H��0���ﵽƽ��ʱ���������ʵ�����Ϊԭ����0.85������ش��������⡣
2Z��g������H��0���ﵽƽ��ʱ���������ʵ�����Ϊԭ����0.85������ش��������⡣
��1��ƽ��ʱZ�����ʵ���Ϊ___________������Ӧ����5min�ﵽƽ�⣬��Y��ƽ����Ӧ����Ϊ____________��
��2����ͬ�����½��з�Ӧ����t1ʱ�̣�ֻ�ı�����ijһ��������������ͼ����ͼ�ס�
A������ B������ C����ѹ D����ѹ E���Ӵ���
��c=0.90mol��t1ʱ�̸ı��������_________����ѡ���ţ���ͬ����t2_______5min (���������������=����ͬ����
��3���������������䣬ԭ����Ϊ��ѹ�������ﵽƽ���Z�����ʵ���______0.9mol (���������������=������
��4���������ʵ�����Ϊ3.00mol����X��Y�����5L�����з�����Ӧ���ڷ�Ӧ������Z�����ʵ����������¶ȱ仯��ͼ�ҡ�
��A��B����Z��������Ӧ���ʵĴ�С��ϵ��_________��
���¶�T��T0ʱ��Z%�������ԭ����______________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com