

���� ���������νӴ�����������ױ�����Ϊ�����ζ����ʣ��ⶨij����������Ʒ�Ĵ��ȣ�ȡa g��Ʒ����ˮ�����Һ�����������������ữ����ȥ�������ƣ��ټ��������������Ȼ�����Һ��������ȫ�������������ȫ�����������ˡ�ϴ�ӡ�����Ƶij�������Ϊb gΪ���ᱵ������
��1��������֪����i��������Ϊ���ᣬ����ii�����õij�����Ϊ�Ȼ�����Һ��
��2��������ϴ���ļ��鷽����ȥ�ϲ���Һ�����Ƿ��������ӣ�
��3�����Ԫ���غ�������������ʵ�����������
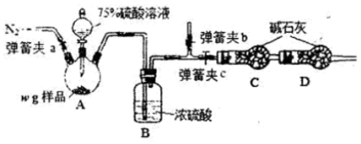
�����巨����ͼʵ��װ��
��4��װ����Ũ������������Ʒ�Ӧ���ɶ�����������ͨ��װ��B��Ũ�������ͨ���������գ�װ��D�Ƿ�ֹ�����ж�����̼��ˮ�������룬��ʼʱ�õ����ž�װ���ڿ�������ֹ�����������������ⶨ����Ӧ�����ͨ�뵪����
��5��Ũ�����ӷ����ɶ��������Ȼ������壬�����D�������Ƿ�ֹ�����ж�����̼��ˮ����������Ųⶨ������������������
�����
����������ϡ���ᷴӦ��������SO2��Ϊ��ֹҺ�����ʱ���У�һ��ɼ������Ƭ������ʵ�飬�迼���Сʵ�������ͨ��N2�ɽ����ɵ�SO2ȫ���ϳ�����֤������Һȫ�����գ�����������Һ���գ������������Һ�������Եõ���ҺB���õⵥ�ʵζ���������ݴ�����
��6�����Һ�ܸ�ʴ��Ӧ����ʽ�ζ��ܣ���ҺBΪ�������λ������������Σ����ο��Ա��ⵥ������Ϊ�����Σ��������۱���ɫ��
��7������c�����⣩=$\frac{c������V������}{V�����⣩}$����������������V��������Ӱ�죬�Դ��жϣ�
��8����Ӧ�����ӷ���ʽΪH2O+SO32-+I2=SO42-+2H++2I-��SO2��SO32-��I2����϶�����ϵ���㣮
��� �⣺���������νӴ�����������ױ�����Ϊ�����ζ����ʣ��ⶨij����������Ʒ�Ĵ��ȣ�ȡa g��Ʒ����ˮ�����Һ�����������������ữ����ȥ�������ƣ��ټ��������������Ȼ�����Һ��������ȫ�������������ȫ�����������ˡ�ϴ�ӡ�����Ƶij�������Ϊb gΪ���ᱵ������
��1��������֪����i��������Ϊ���ᣬ������Ϊ�Ȼ�����Һ���ʴ�Ϊ�����BaCl2��Һ��
��2��������ϴ���ļ��鷽����ȥ�ϲ���Һ�����Ƿ��������ӣ�ȡ�������һ��ϴ��Һ���Թ��У�����AgNO3��Һ������������֤��ϴ�Ӹɾ���
�ʴ�Ϊ��ȡ�������һ��ϴ��Һ���Թ��У�����AgNO3��Һ������������֤��ϴ�Ӹɾ���
��3��b gΪ���ᱵ�������ʵ���n=$\frac{bg}{233g/mol}$=$\frac{b}{233}$mol�����Ԫ���غ�������������ʵ���������=$\frac{\frac{b}{233}mol��142gmol}{ag}$��100%=$\frac{142b}{233a}$��100%��
�ʴ�Ϊ��$\frac{142b}{233a}$��100%��
�����巨����ͼʵ��װ��
��4��װ����Ũ������������Ʒ�Ӧ���ɶ�����������ͨ��װ��B��Ũ�������ͨ���������գ�װ��D�Ƿ�ֹ�����ж�����̼��ˮ�������룬��ʼʱ�õ����ž�װ���ڿ�������ֹ�����������������ⶨ����Ӧ�����ͨ�뵪����ʵ�鿪ʼʱ������Ҫ�رյ��ɼ�c���ٴ��ɼ�a��b����װ����ͨ��N2��ͨN2���������ų�װ���ڿ��������ӿ�����������������̼����ʵ�飬��װ��A�з�Ӧ������Ϊʹ��Ӧ�����ɵ�SO2��װ��C�еļ�ʯ����ȫ���գ���Ҫ���еIJ���Ϊ���ٴ�ͨ�뵪����
�ʴ�Ϊ���ų�װ���ڿ��������ӿ�����������������̼����ʵ�飻�ٴ�ͨ�뵪����
��5��Ũ�����ӷ����ɶ��������Ȼ������壬������Ũ�������75%��������Һ�������D�������Ƿ�ֹ�����ж�����̼��ˮ����������Ųⶨ������������������
�ʴ�Ϊ����ֹ�����ж�����̼��ˮ��������װ��C��
�����
��6�����Һ�ܸ�ʴ�������ü�ʽ�ζ��ܣ�������ʽ�ζ��ܣ��������λ���������������ⵥ�ʷ�Ӧ��HSO3-+I2+H2O=SO42-+2I-+3H+���������۱���ɫ���õ����Һ�ζ��������λ������������Σ�����ʹ�õ�����Һ��ָʾ��������ɫ��ʧʱ����Ӧǡ�÷��������ﵽ�յ�ʱ������Ϊ����Һ����ɫ��Ϊ��ɫ���Ұ�����ڲ���ɫ��
�ʴ�Ϊ���������Һ��
��7��δ�ñ�Һ��ϴ�ζ��ܣ������V������ƫ����c�����⣩=$\frac{c������V������}{V�����⣩}$�����������c�����⣩ƫ�ߣ�
�ʴ�Ϊ��ƫ�ߣ�
��8����Ӧ�����ӷ���ʽΪH2O+SO32-+I2=SO42-+2H++2I-��
SO2��SO32-��I2��
1mol 1mol
n 0.01000mol•L-1��V��10-3L=V��10-5mol
n=V��10-5mol��
��Ʒ�к�Na2SO3�Ĵ���=$\frac{126g/mol��V��1{0}^{-5}mol}{wg}$��100%=$\frac{0.126V}{w}$%��
�ʴ�Ϊ��$\frac{0.126V}{w}$%��
���� ��������ҵ��������ʳƷ�е��������κ����IJⶨΪ�����龳��ͨ��������ʵ������ͼ����ʵ�����������������ʹ�á���ѧ���㡢��ѧ����ʽ����д�������ۺ���һ�𣬿��鿼���Ի�ѧʵ�鷽���ķ���������������������Ŀ�Ѷ��еȣ�



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ��
�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� |  | B�� |  | C�� | 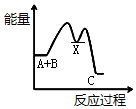 | D�� | 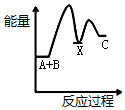 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| A�� | pH=2��HA��Һ��pH=12��MOH��Һ����Ȼ�ϣ�c��H+��+c��M+���Tc��OH-��+c��A-�� | |
| B�� | 1 L 0.1 mol•L-1 ��NH4��2Fe��SO4��2����Һ�У�c��SO${\;}_{4}^{2-}$����c��NH${\;}_{4}^{+}$����c��Fe2+����c��H+����c��OH-�� | |
| C�� | 0.1 mol•L-1 NaHCO3��Һ�У�c��H+��+c��H2CO3���Tc��CO${\;}_{3}^{2-}$��+c��OH-�� | |
| D�� | 0.1mol•L-1��NaHA��Һ����pH=4��c��HA-����c��H+����c��H2A����c��A2-�� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
| ѡ�� | ʵ��Ŀ�� | ʵ�鷽�� |
| A | ֤����84������Һ��������������ҺpH�ļ�С����ǿ | ����84������Һ����NaClO������Ʒ����Һ�У���ɫ��������ͬʱ����ʳ�ף���ɫ�ܿ���Ϊ��ɫ |
| B | ֤����Ӧ���ʻ��淴Ӧ��Ũ�ȵ�������ӿ� | ��3mLϡ������������п��Ӧ�������������ʽ�����Ȼ�����1mL1mol•L-1CuSO4��Һ��Ѹ�ٲ����϶����� |
| C | ��������Ƿ�ˮ�� | ���Թ���ȡ�������ۣ�����ϡ��������Ƭ�̣���ȴ��ȡ����Һ�������Ƶ�Cu��OH��2���ȷ��� |
| D | ֤��SO2��Ư���� | ��SO2ͨ�����Ը��������Һ�� |
| A�� | A | B�� | B | C�� | C | D�� | D |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
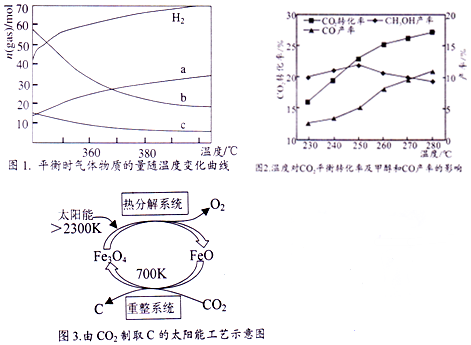
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�ѡ����
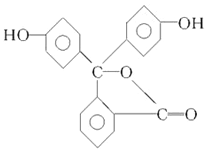
| A�� | ��̪�����к������ֹ����� | |
| B�� | ��̪�ķ���ʽΪC20H12O4 | |
| C�� | ��̪���ڷ����� | |
| D�� | ��̪�ṹ�к����ǻ���-OH�����ʷ�̪���ڴ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com