
ij��ѧ�о���ѧϰС��ͨ���������ϣ����������ͼ��ʾ�ķ����Ժ����ϴ���Ϊԭ�����Ʊ�NiSO4??7H2O����֪ij�������ĺ���������Ҫ����Ni��������Al��31%����Fe��1.3%���ĵ��ʼ������������������(3.3%)��
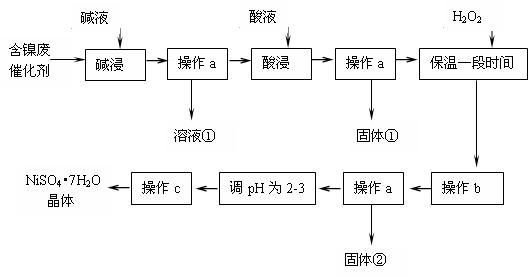
����������������������ʽ��ȫ����ʱ��pH���£�
| ������ | Al(OH)3 | Fe(OH)3 | Fe(OH)2 | Ni(OH)2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
�ش��������⣺
�� ����a��c����ʹ�õ�����������̨������Ȧ�����ƾ��ơ��ձ������������Ҫ����Ҫ����Ϊ ��
�� ������������з��������ӷ���ʽ�� ��
�ǡ������ʱ����������� (�ѧʽ�������������a���������ٺ���Һ�п��ܺ��еĽ��������� ��
�� ����bΪ������Һ��pH������ΪpH����ѵ��ط�Χ�� ��
�ɡ���pHΪ2~3����Ŀ���� ��
�� ��Ʒ��������ʱ����������̷���FeSO4��7H2O������ԭ������� ��
 ����ͬ�����Ծ�ϵ�д�
����ͬ�����Ծ�ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
 ij��ѧ�о���ѧϰС���ij��ɫˮ���ijɷֽ��м��飬��֪��ˮ����ֻ���ܺ���K+��Mg2+��Fe3+��Cu2+��Al3+��Ag+��Ca2+��CO
ij��ѧ�о���ѧϰС���ij��ɫˮ���ijɷֽ��м��飬��֪��ˮ����ֻ���ܺ���K+��Mg2+��Fe3+��Cu2+��Al3+��Ag+��Ca2+��CO2- 3 |
2- 4 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

| ������ | Al��OH��3 | Fe��OH��3 | Fe��OH��2 | Ni��OH��2 |
| pH | 5.2 | 3.2 | 9.7 | 9.2 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?̩��һģ��ij��ѧ�о���ѧϰС��Ϊ̽��ijƷ�ƻ������в�����֬����ĺ���������������ʵ�飺
��2011?̩��һģ��ij��ѧ�о���ѧϰС��Ϊ̽��ijƷ�ƻ������в�����֬����ĺ���������������ʵ�飺
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�

�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com