
£Ø15·Ö£© I ŌŚĻĀĶ¼×Ŗ»Æ¹ŲĻµÖŠ£¬¹ĢĢå¼×µÄŃęÉ«·“Ó¦³Ź»ĘÉ«£¬MĪŖ³£¼ūµÄŅŗĢåĪļÖŹ£¬ĖįGŹĒÖŲŅŖµÄ»Æ¹¤²śĘ·ŗĶ»Æ¹¤ŌĮĻ£»¹ĢĢåHÄÜČܽāŌŚAŗĶĖįGĒŅHĪŖĮ¼ŗƵÄÄĶ»š²ÄĮĻ(Ķ¼ÖŠ²æ·Ö²śĪļƻӊĮŠ³ö)”£

IIČēĶ¼±ķŹ¾µÄŹĒÉś²śĖįGµÄ¹¤ŅµĮ÷³Ģ£ŗ

£Ø1£©ŅŅÉč±øµÄĆū³ĘĪŖ ¹ĢĢåŅŅµÄ»ÆѧŹ½ĪŖ M·Ö×ӵĽį¹¹Ź½ĪŖ ”£
£Ø2£©ĘųĢåXµÄÖ÷ŅŖ³É·ÖĪŖ ”£
£Ø3£©Š“³öÉč±ø¼×ÖŠ³£¼ūµÄ»Æѧ·“Ó¦ ”£
£Ø4£©°×É«³ĮµķDÓėGČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø5£©½«2.24 L£Ø±ź×¼×“æöĻĀ£©EĶØČė100 mL2 mol/L AµÄĖ®ČÜŅŗŗó£¬ČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ____________ ___”£
£Ø6£©µē½āČŪČŚ¹ĢĢåHæÉÖʵĆŅ»ÖÖ½šŹō£¬Ōņµē½ā»Æѧ·½³ĢŹ½ĪŖ µ±µē½āŹ±×ŖŅĘ2.4molµē×Ó£¬ÖĘµĆ½šŹō g”£
£Ø1£©½Ó“„ŹŅ£Ø1·Ö£© Al2S3 £Ø1·Ö£© H”Ŗ”ŖO”Ŗ”ŖH £Ø1·Ö£©
£Ø2£©SO2”¢O2”¢N2£Ø2·Ö£©
£Ø3£©4FeS2 + 11O2  2Fe2O3
+ 8SO2£Ø2·Ö£¬ĘäĖūŗĻĄķŅ²øų·Ö£©
2Fe2O3
+ 8SO2£Ø2·Ö£¬ĘäĖūŗĻĄķŅ²øų·Ö£©
£Ø4£©Al(OH)3 + 3H+ === Al3+ + 3H2O £Ø2·Ö£©
£Ø5£©c(Na*)£¾c(SO32-)£¾c(OH-)£¾c(HSO3-)£¾c(H*) £Ø2·Ö£©
£Ø6£©2Al2O3 4 Al + 3O2”ü(Ö»ŅŖÓŠĶصē»ņµē½āĢõ¼ž¼“æÉ) £Ø2·Ö£©
21.6g£Ø2·Ö£©
4 Al + 3O2”ü(Ö»ŅŖÓŠĶصē»ņµē½āĢõ¼ž¼“æÉ) £Ø2·Ö£©
21.6g£Ø2·Ö£©
”¾½āĪö”æ



| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
£Ø15·Ö£© I ŌŚĻĀĶ¼×Ŗ»Æ¹ŲĻµÖŠ£¬¹ĢĢå¼×µÄŃęÉ«·“Ó¦³Ź»ĘÉ«£¬MĪŖ³£¼ūµÄŅŗĢåĪļÖŹ£¬ĖįGŹĒÖŲŅŖµÄ»Æ¹¤²śĘ·ŗĶ»Æ¹¤ŌĮĻ£»¹ĢĢåHÄÜČܽāŌŚAŗĶĖįGĒŅHĪŖĮ¼ŗƵÄÄĶ»š²ÄĮĻ(Ķ¼ÖŠ²æ·Ö²śĪļƻӊĮŠ³ö)”£
IIČēĶ¼±ķŹ¾µÄŹĒÉś²śĖįGµÄ¹¤ŅµĮ÷³Ģ£ŗ
£Ø1£©ŅŅÉč±øµÄĆū³ĘĪŖ ¹ĢĢåŅŅµÄ»ÆѧŹ½ĪŖ M·Ö×ӵĽį¹¹Ź½ĪŖ ”£
£Ø2£©ĘųĢåXµÄÖ÷ŅŖ³É·ÖĪŖ ”£
£Ø3£©Š“³öÉč±ø¼×ÖŠ³£¼ūµÄ»Æѧ·“Ó¦ ”£
£Ø4£©°×É«³ĮµķDÓėGČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø5£©½«2.24 L£Ø±ź×¼×“æöĻĀ£©EĶØČė100mL2 mol/L AµÄĖ®ČÜŅŗŗó£¬ČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ____________ ___”£
£Ø6£©µē½āČŪČŚ¹ĢĢåHæÉÖʵĆŅ»ÖÖ½šŹō£¬Ōņµē½ā»Æѧ·½³ĢŹ½ĪŖ µ±µē½āŹ±×ŖŅĘ2.4molµē×Ó£¬ÖĘµĆ½šŹō g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2012½ģɽ¶«Ź”Ēśø·Ņ»ÖŠøßČżµŚŅ»“ĪĆžµ×æ¼ŹŌ»ÆѧŹŌ¾ķ ĢāŠĶ£ŗĢīæÕĢā
£Ø12·Ö£©ŅŃÖŖŌŚĻĀĶ¼×Ŗ»Æ¹ŲĻµ£ØijŠ©×Ŗ»Æ¹ŲĻµÖŠµÄ²śĪļŅŃĀŌČ„£©ÖŠ¶¼ŹĒ֊ѧ»Æѧ³£¼ūµÄĪļÖŹ£¬ĪļÖŹA”¢D”¢GŹĒµ„ÖŹ£¬“ÅŠŌŗŚÉ«ĪļÖŹCŹĒij½šŹōæóĪļµÄÖ÷ŅŖ³É·Ż£¬EŹĒŅ»ÖÖ·ĒŃõ»ÆŠŌĖį£¬FŹĒ»ģŗĻĪļ£¬HŹĒ¼«Ņ×ČÜÓŚĖ®µÄ¼īŠŌĘųĢ唣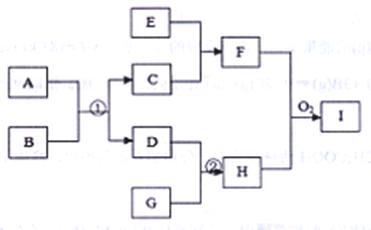
Ēė»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©Š“³öĻĀĮŠĪļÖŹµÄ»ÆѧŹ½£ŗA ”¢C ”¢I
£Ø2£©Š“³ö·“Ó¦¢ŁµÄ»Æѧ·½³ĢŹ½£ŗ
Š“³öÉś³É»ģŗĻĪļFµÄĄė×Ó·½³ĢŹ½£ŗ
£Ø3£©ŌŚŅ»¶ØĪĀ¶Č”¢Ń¹ĒæŗĶÓŠ“߻ƼĮ“ęŌŚµÄĢõ¼žĻĀ½«l mol GŗĶ2,5 mol D·ÅČė500mLĆܱÕČŻĘ÷ÖŠ”£¾¹ż20min“ļµ½Ę½ŗā£¬Ę½ŗāŗóHµÄÅضČĪŖ2mol/L£¬
¢ŁŌņÓĆG±ķŹ¾20minÄŚµÄĘ½¾ł·“Ó¦ĖŁĀŹĪŖ£ŗ____
øĆĪĀ¶ČĻĀ“Ė·“Ó¦µÄĘ½ŗā³£ŹżK= £¬DµÄ×Ŗ»ÆĀŹĪŖ
¢ŚČē¹ū±£³ÖĪĀ¶Č²»±ä£¬ŌŁĻņČŻĘ÷ÖŠĶ¬Ź±³äČė1£®5 mol GŗĶ1 mol H£¬DµÄ×Ŗ»ÆĀŹ½« £ØĢī”°Éżøß”±”¢”°²»±ä”±»ņ”°½µµĶ”±£©£¬ŌŁ“ĪĘ½ŗāŗóHµÄĢå»ż·ÖŹżĪŖ____ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ2010ÄźøŹĖąŹ”²æ·ÖĘÕĶØøßÖŠøßČżµŚ¶ž“ĪĮŖŗĻæ¼ŹŌ£ØĄķ×Ū£©»Æѧ²æ·Ö ĢāŠĶ£ŗĢīæÕĢā
£Ø15·Ö£©IŌŚĻĀĶ¼×Ŗ»Æ¹ŲĻµÖŠ£¬¹ĢĢå¼×µÄŃęÉ«·“Ó¦³Ź»ĘÉ«£¬MĪŖ³£¼ūµÄŅŗĢåĪļÖŹ£¬ĖįGŹĒÖŲŅŖµÄ»Æ¹¤²śĘ·ŗĶ»Æ¹¤ŌĮĻ£»¹ĢĢåHÄÜČܽāŌŚAŗĶĖįGĒŅHĪŖĮ¼ŗƵÄÄĶ»š²ÄĮĻ(Ķ¼ÖŠ²æ·Ö²śĪļƻӊĮŠ³ö)”£
IIČēĶ¼±ķŹ¾µÄŹĒÉś²śĖįGµÄ¹¤ŅµĮ÷³Ģ£ŗ
£Ø1£©ŅŅÉč±øµÄĆū³ĘĪŖ ¹ĢĢåŅŅµÄ»ÆѧŹ½ĪŖ M·Ö×ӵĽį¹¹Ź½ĪŖ ”£
£Ø2£©ĘųĢåXµÄÖ÷ŅŖ³É·ÖĪŖ ”£
£Ø3£©Š“³öÉč±ø¼×ÖŠ³£¼ūµÄ»Æѧ·“Ó¦ ”£
£Ø4£©°×É«³ĮµķDÓėGČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø5£©½«2.24 L£Ø±ź×¼×“æöĻĀ£©EĶØČė100 mL2 mol/L AµÄĖ®ČÜ Ņŗŗó£¬ČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ____________ ___”£
Ņŗŗó£¬ČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ____________ ___”£
£Ø6£©µē½āČŪČŚ¹ĢĢåHæÉÖʵĆŅ»ÖÖ½šŹō£¬Ōņµē½ā»Æѧ·½³ĢŹ½ĪŖ µ±µē½āŹ±×ŖŅĘ2.4molµē×Ó£¬ÖĘµĆ½šŹō g”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
I ŌŚĻĀĶ¼×Ŗ»Æ¹ŲĻµÖŠ£¬¹ĢĢå¼×µÄŃęÉ«·“Ó¦³Ź»ĘÉ«£¬MĪŖ³£¼ūµÄŅŗĢåĪļÖŹ£¬ĖįGŹĒÖŲŅŖµÄ»Æ¹¤²śĘ·ŗĶ»Æ¹¤ŌĮĻ£»¹ĢĢåHÄÜČܽāŌŚAŗĶĖįGĒŅHĪŖĮ¼ŗƵÄÄĶ»š²ÄĮĻ(Ķ¼ÖŠ²æ·Ö²śĪļƻӊĮŠ³ö)”£

IIČēĶ¼±ķŹ¾µÄŹĒÉś²śĖįGµÄ¹¤ŅµĮ÷³Ģ£ŗ

£Ø1£©ŅŅÉč±øµÄĆū³ĘĪŖ ¹ĢĢåŅŅµÄ»ÆѧŹ½ĪŖ M·Ö×ӵĽį¹¹Ź½ĪŖ ”£
£Ø2£©ĘųĢåXµÄÖ÷ŅŖ³É·ÖĪŖ ”£
£Ø3£©Š“³öÉč±ø¼×ÖŠ³£¼ūµÄ»Æѧ·“Ó¦ ”£
£Ø4£©°×É«³ĮµķDÓėGČÜŅŗ·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ”£
£Ø5£©½«2.24 L£Ø±ź×¼×“æöĻĀ£©EĶØČė100 mL2 mol/L AµÄĖ®ČÜŅŗŗó£¬ČÜŅŗÖŠĄė×ÓÅضČÓɓ󵽊”µÄĖ³ŠņĪŖ____________ ___”£
£Ø6£©µē½āČŪČŚ¹ĢĢåHæÉÖʵĆŅ»ÖÖ½šŹō£¬Ōņµē½ā»Æѧ·½³ĢŹ½ĪŖ µ±µē½āŹ±×ŖŅĘ2.4molµē×Ó£¬ÖĘµĆ½šŹō g”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com