�⣺��1������������������ǵ�������Ͷ������ʴ�Ϊ��NO��NO
2��SO
2��
��2��������CԪ�صĻ��ϼ�Ϊ+4�ۣ���Ӧ��ֻ��NԪ�ر�ۣ�4molCO��NH
2��
2��-3�۵�Nʧȥ24mol���ӣ�6molNO
2��+4�۵�N�õ�24mol���ӣ�����7mol N
2��ת��24mol���ӣ���12g�����ʵ���=

=0.2mol����������12g����ʱ����ʱ����ת��1.2 mol���ӣ��ʴ�Ϊ��1.2 mol��
��3��6NO��g��+4NH
3��g��?5N
2��g��+6H
2O��g����H
1=-1627.2kJmol
-1��
4NO��g��+4NH
3��g��+O
2��g��?4N
2��g��+6H
2O��g����H
2=-18070.0kJmol
-1 ��
6NO��g��+8NH
3��g��?7N
2��g��+12H
2O��g����H
3=-2659.9kJmol
-1��
������ʽ��-�ٵ�N
2��g��+O
2��g��?2NO��g����H=��H
2-��H
1=-18070.0kJmol
-1-��-1627.2kJmol
-1��=-179.8kJ/mol��
�ʴ�Ϊ��-179.8��
��4����ͼ���֪���¶�����ƽ�������ƶ������ԡ�H��0��T
1ʱ��A���Ӧ�Ħ�=0.5����ƽ�ⳣ������ʽ�ɵ�K=1����B����A���¶���ͬ��K���䣬�ɼ�������Ϊ2L���ʴ�Ϊ������2��
��5����NH
4��
2SO
4��Һ��NH
4+ˮ�������ԣ�������Ũ�ȴ�С˳��Ϊc��NH
4+����c��SO
42-����c��H
+����c��OH
-����
0.05mol?L
-1��NH
4��
2SO
4��Һ��pH=a����c��OH
-��=10
a-14��Kb=

1.7��10
-5mol��L
-1������

=

=1.7��10
9-a��
�ʴ�Ϊ��c��NH
4+����c��SO
42-����c��H
+����c��OH
-����1.7��10
9-a��
��������1������������������ǵ�������Ͷ�������
��2���������غ�ת�Ƶ���֮��Ĺ�ϵʽ���㣻
��3�����ݸ�˹���ɽ��
��4�������¶Ⱥ�CO��ת����ȷ����Ӧ�ȣ��¶Ȳ��䣬��ѧƽ�ⳣ�����䣬����CO��ת����ȷ����Ӧ���Ӷ�ȷ������������仯��
��5�������ε����ͼ��εĻ�ѧʽȷ������Ũ�ȴ�С������һˮ�ϰ��ĵ��볣������Һ������������Ũ�ȼ��㣮
�������������о���Ⱦ�������к���������Ϊ���忼��������ԭ��Ӧ���Ȼ�ѧ��Ӧ����ѧƽ�⼰�������Һ�����֪ʶ���Ѷ��еȣ�

 CO��NO��NO2��SO2�ȶ�����Ⱦ�������к����壬������л��������ǽ��ܼ��ŵ���Ҫ���⣮
CO��NO��NO2��SO2�ȶ�����Ⱦ�������к����壬������л��������ǽ��ܼ��ŵ���Ҫ���⣮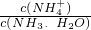 =______���ú�a�Ĵ���ʽ��ʾ����֪NH3��H2O�ĵ��볣��Kb=1.7��10-5mol��L-1����
=______���ú�a�Ĵ���ʽ��ʾ����֪NH3��H2O�ĵ��볣��Kb=1.7��10-5mol��L-1���� =0.2mol����������12g����ʱ����ʱ����ת��1.2 mol���ӣ��ʴ�Ϊ��1.2 mol��
=0.2mol����������12g����ʱ����ʱ����ת��1.2 mol���ӣ��ʴ�Ϊ��1.2 mol�� 1.7��10-5mol��L-1������
1.7��10-5mol��L-1������ =
= =1.7��109-a��
=1.7��109-a��

 �ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�
�ִʾ�ƪ��ͬ�����Ĵ��ϵ�д�

