
�ס��ҡ���Ϊ�������ʡ�A��B��C��D��E��F��G��H��Ϊ��ѧ��ѧ�г����Ļ��������B��G����ɫ��Ӧ��Ϊ��ɫ��C��ʹƷ����Һ��ɫ����һ�������£��������ת����ϵ��ͼ��ʾ������֪E�ĵ��������������������

��ش��������⣺
��1���û�ѧʽ��ʾ����Ϊ__________��HΪ__________��
��2��A�ĵ���ʽΪ________________________________��
��3����E��Һ��ͨ�����C�Ļ�ѧ��Ӧ����ʽΪ_____________________��
��4��д��B��C D�Ļ�ѧ����ʽ��_________________________________
D�Ļ�ѧ����ʽ��_________________________________
д��E��G F�����ӷ���ʽ��______________________________________
F�����ӷ���ʽ��______________________________________

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭���߰���ѧ��һ�����л�ѧ�����ص�ࣩ�������棩 ���ͣ�ѡ����
���и���ѡ��յ���ʡ����ǵ���ʡ������ʡ��������˳�����е�һ���ǣ� ��
A��HCl��SO3��ʯī��Һ�� B��NaCl���Ҵ�����������
C�����ǡ�CuSO4��������������Һ D��KCl��NaNO3��������ʯ��ˮ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ��ӱ�ʡ��һ�ϵ�һ���¿���ѧ�Ծ��������棩 ���ͣ�ѡ����
����CuSO4��Һ��Fe(OH)3����������ȷ����
A. ���߶��ܲ������������
B�����߶����ܲ������������
C��CuSO4��Һ�ܲ������������Fe(OH)3���岻�ܲ������������
D. CuSO4��Һ���ܲ������������Fe(OH)3�����ܲ������������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ����������������и�һ�����в��Ի�ѧ�Ծ��������棩 ���ͣ������
�������ʣ���Cu �� CO2 �� H2SO3 �� H2O �� Һ̬HCl �� H2SO4 �� Ba(OH)2 �� NaCl ������ �� NaOH��Һ��
���ڵ���ʵ��� �����ڷǵ���ʵ��� ���ܵ������ ��������ţ�������ȫ�����÷֣�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ��һ�ϵڶ��ζο���ѧ�Ծ��������棩 ���ͣ�ʵ����
������������ʵķ����ᴿ���⡣
�����к�NaCl��Na2SO4��NaNO3�Ļ���ѡ���ʵ����Լ���ȥ��Һ�е�NaCl��Na2SO4���Ӷ��õ�������NaNO3��Һ����Ӧ��ʵ����̿�����ͼ��ʾ��
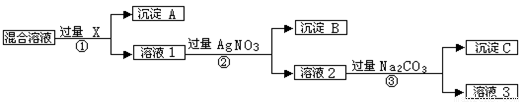
��ش��������⣺
��1��д��ʵ���������������ʵĻ�ѧʽ���Լ�X_______������B ��
��2��֤��AgNO3�ӹ�����ʵ�鷽���� ��
��3������ʵ�鷽���õ�����Һ3�п϶�����__________���ѧʽ�����ʣ�Ϊ�˽��������⣬��������Һ3�м���������___________��֮����Ҫ��ù���NaNO3����е�ʵ�������___________����������ƣ���
��ijͬѧ����CCl4��ȡ�ϸ�Ũ�ȵĵ�ˮ�еĵ⣬�������̿��Էֽ�Ϊ���¼�����
A����ʢ����Һ�ķ�Һ©����������̨����Ȧ�У�
B����50ml��ˮ��15mlCCl4�����Һ©���У����Ǻò�������
C�������Һ©���������ϿڵIJ������Ƿ�©Һ��
D����ת©������������ʱ�����������������رջ������ѷ�Һ©��������
E���������������ձ�������Һ��
F���ӷ�Һ©���Ͽڵ����ϲ�Һ�壻
G����©���ϿڵIJ�������ʹ���ϵİ�����©���Ͽڵ�С����
H�����á��ֲ㡣
��1����ȡ������ȷ���������˳���ǣ���������ĸ�� ��
��2�����һ�ּ�����ȡ����ˮ��Һ���Ƿ��е��ʵ�ļ����� ��[��Դ:Z��3�������CCl4��ͨ�� ��ã��©���Ͽڡ���©���¿ڡ������Ӻ����CCl4��Һ����ȡ�� �ͻ���CCl4������Ҫ�������۲���ͼ��ʾʵ��װ��ָ��������� ����
��4�����������������ʱ�����̬����____________�����������ƣ���ۼ���

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������������ѧ�߶���ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ������
һ�ܱ���ϵ�з������з�Ӧ��N2(g����3H2(g�� 2NH3(g�� ��H��0����ͼ��ijһʱ����з�Ӧ�����뷴Ӧʱ������߹�ϵͼ���ش��������⣺
2NH3(g�� ��H��0����ͼ��ijһʱ����з�Ӧ�����뷴Ӧʱ������߹�ϵͼ���ش��������⣺
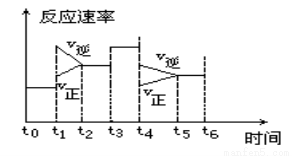
��1������ƽ��״̬��ʱ�����_________________
��2��t1��t3��t4ʱ����ϵ�зֱ���ʲô���������˱仯��________________________��
��3�����и�ʱ����ڣ����İٷֺ�����ߵ���
A��t0��t1 B��t2��t3 C��t3��t4 D��t5��t6
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ������������ѧ�߶���ѧ�����в��Ի�ѧ�Ծ��������棩 ���ͣ�ѡ����
��֪2H2(g����O2(g�� =2H2O(l�� ��H����569.6kJ/ mol,
mol,
2H2O(g��=2H2(g����O2(g�� ��H����482.1 kJ/mol��
����1 gҺ̬H2O������ʱ���յ�������
A��2.43 kJ B��4.86 kJ C��43.8 kJ D��87.5 kJ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�긣��ʡ�����и߶������У�������ѧ�Ծ��������棩 ���ͣ�ѡ����
��һ����ƽ��״̬�ķ�Ӧ��X(s)��3Y(g) 2Z(g) ��H<0��Ϊ��ʹƽ��������Z�ķ����ƶ���Ӧѡ���������
2Z(g) ��H<0��Ϊ��ʹƽ��������Z�ķ����ƶ���Ӧѡ���������
A������X���� B��ʹ�ô��� C�������¶� D������ѹǿ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015-2016ѧ�꽭��ʡ��ʿ���ɻ���ɽ����У�߶������б���ѧ�Ծ��������棩 ���ͣ�ѡ����
����ʵ���У�ʼ���������������
A��NH3ͨ��AlCl3��Һ��
B��SO2ͨ��HNO3�ữ��Ba(NO3)2��Һ��
C��NO2ͨ��FeSO4��Һ��
D��CO2ͨ��CaCl2��Һ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com