
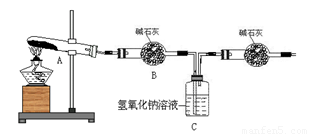
��17�֣�����ͭ���ȷֽ���������ͭ�����壬�����¶Ȳ�ͬ��������ɷ�Ҳ��ͬ������ɷֿ��ܺ�SO2��SO3��O2�е�һ�֡����ֻ����֡�ij��ѧ����С��ͨ�����һ̽����ʵ�飬̽���ⶨ��Ӧ������SO2��SO3��O2�����ʵ�������������ȷ�������ʵĻ�ѧ���������Ӷ�ȷ��CuSO4�ֽ�Ļ�ѧ����ʽ��ʵ������õ�����������ͼ��ʾ��
��������롿
����������ijɷֿ���ֻ�� һ�֣�
����������ijɷֿ��ܺ��� ���֣�
����������ijɷֿ��ܺ��� ���֡�
��ʵ��̽����
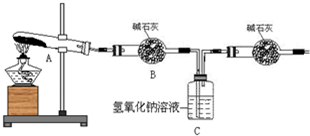
ʵ��������̣��ԣ�����֪ʵ�����ʱ������ͭ��ȫ�ֽ⡣��ش��������⣺
(1) ������װ̽��ʵ���װ�ã����������ҵķ��������ӿ�����˳��Ϊ��
__ (�����)
(2) ��ʵ�������B����Ͳû���ռ���ˮ����֤������ ��ȷ��
��3����ʵ����������ⶨװ��C�������������ˣ��ܷ�϶���������к���SO2������SO3��
��˵�����ɡ���
��4��������ʵ��С����и�ʵ�飬���ڼ���ʱ���¶Ȳ�ͬ,ʵ���������������Ҳ��ͬ,�������£�
| ʵ��С�� | ��ȡCuSO4 ��������g�� | װ��C���� ������(g) | ��Ͳ��ˮ���������ɱ� ״������������(mL) |
| һ | 6.4 | 2.56 | 298.7 |
| �� | 6.4 | 2.56 | 448 |
��ͨ�����㣬�ƶϳ���һС��͵ڶ�С���ʵ��������CuSO4�ֽ�Ļ�ѧ��Ӧ����ʽ��
һ�飺_____________ ��
���飺_ ��
(5)��ʵ������У�����ʵ�������ԭ�������ⶨ���������������������________��_______(�����)��

| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2013?բ������ģ��������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
��2013?բ������ģ��������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺| װ�� | A ���Թ�+��ĩ�� |
B | C |
| ��Ӧǰ | 42.0g | 75.0g | 140.0g |
| ��Ӧ�� | 37.0g | 79.0g | 140.5g |
| ���� |
| ���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ���բ����������ѧ�ڶ�ģ��ѧ�Ծ��������棩 ���ͣ�������
������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
��1����ͭ���ǹ�ҵ��ͭ����Ҫԭ�ϣ�����Ҫ�ɷ�ΪCuFeS2��
�ٲ��ij��ͭ��(CuFeS2)�к���20%����������������ÿ�ʯ��ͭ������������
������һ����Ȼ��ͭ��������ʯ����Ϊ�˲ⶨ�û�ͭ��Ĵ��ȣ�ijͬѧ���������ʵ�飺��ȡ
��ϸ�Ļ�ͭ����Ʒ1.150g���ڿ����н������գ�����Cu��Fe3O4��SO2���壬��100 mL���е��۵�
����ˮȫ������SO2��Ȼ��ȡ10mL����Һ����0.05mol/L������Һ���еζ�����ȥ������Һ����
��Ϊ20.00mL����û�ͭ��Ĵ��ȡ�
��2����FeS��Fe2O3�Ļ����56.6 g��������ϡH2SO4�ܽ��ɵ�3.2 g��ԭ�������FeS��������
��3��һ���¶��£�����ͭ���ȷֽ�����CuO��SO2��SO3��O2����֪��SO2��SO3���ܱ���ʯ�Һ�����
������Һ���ա�������ͼװ�ü�����ˮ����ͭ��ĩֱ����ȫ�ֽ⡣����ˮ����ͭ��ĩ����Ϊ10.0 g��
��ȫ�ֽ��װ�õ������仯��ϵ���±���ʾ��
|
װ�� |
A���Թ�+��ĩ�� |
B |
C |
|
��Ӧǰ |
42.0 g |
75.0 g |
140.0 g |
|
��Ӧ�� |
37.0 g |
79.0 g |
140.5 g |
��ͨ�����㣬�ƶϳ���ʵ������������ͭ�ֽ�Ļ�ѧ����ʽ��
��4������������Ƥ�����Ҫ��ѧ�Լ���������ˮNa2SO4��̿���ڸ����·�Ӧ�Ƶã���ѧ����ʽ���£�
��Na2SO4
+ 4C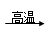 Na2S + 4CO�� ��Na2SO4
+ 4CO
Na2S + 4CO�� ��Na2SO4
+ 4CO Na2S + 4CO2
Na2S + 4CO2
a.���ڷ�Ӧ�����У�����CO��CO2�������Ϊ2mol��������Na2S�����ʵ�����
b.���ƾ�������ڿ����У��Ỻ��������Na2SO3��������Na2SO4���ֽ�43.72g���ֱ��ʵ�������Ʒ����ˮ�У���������������˵�4.8g������1.12L H2S ���壨��״����������Һ������ȫ���ݳ���������Һ�м���������BaCl2����˵�2.33g������������������Ʒ�ijɷּ������ʵ�����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2010�꽭��ʡ�����������������ۺϣ���ѧ���� ���ͣ�ʵ����
��15�֣���ˮ����ͭ�ڼ����������ܷ����ֽⷴӦ����������ͭ�������������������������ijѧ����ͼ����ͼ��ʾװ����ȷ���û�ѧ��Ӧ�и����ʵļ�����ϵ

�Իش�
��1�����ȹ����У��Թ�A�з�����ʵ������Ϊ ��
��2��װ��E��F�������� ��
��3����ѧ��ʹ��װ��B�ı����dz�ȥ��������е������������ᴿ����������������ȷ
��Ϊʲô��
��4����ѧ����������װ�ý�һ����������ˮ����ͭ����A�м���ʹ��ֽ⣬�������
�������ƫС����ԭ������������� ��������ţ�
A����ˮ����ͭδ��ȫ�ֽ�o*m
B��ʵ�����ʱװ��A�в���������
C��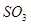 ��
��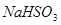 ��Һ����ʱ������
��Һ����ʱ������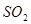 ����
����
D��������Ͳ�еĶ���ʱ��E�е�Һ�����F�е�Һ��
��5����һѧ����4.8g��ˮ����ͭ��ּ���ʹ����ȫ�ֽ������ȷ��ʵ�鷽����ȥ���������еĶ������������������������������Ϊ224mL����״�������ݴ˿ɼ������������Ϊ mol����������Ϊ mol��
��6��������ʵ�����ݿ�֪��ˮ����ͭ���ȷֽ�Ļ�ѧ����ʽΪ��
___________________________________________________________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ������
 ������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺
������Ļ������ڹ�ҵ������Ӧ�ù㷺���ش��������⣺| װ�� | A ���Թ�+��ĩ�� | B | C |
| ��Ӧǰ | 42.0g | 75.0g | 140.0g |
| ��Ӧ�� | 37.0g | 79.0g | 140.5g |
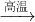 Na2S+4CO�� ��Na2SO4+4CO
Na2S+4CO�� ��Na2SO4+4CO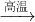 Na2S+4CO2
Na2S+4CO2�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2013���Ϻ���բ�����߿���ѧ��ģ�Ծ��������棩 ���ͣ������
| װ�� | A ���Թ�+��ĩ�� | B | C |
| ��Ӧǰ | 42.0g | 75.0g | 140.0g |
| ��Ӧ�� | 37.0g | 79.0g | 140.5g |
 Na2S+4CO ��Na2SO4+4CO
Na2S+4CO ��Na2SO4+4CO Na2S+4CO2
Na2S+4CO2
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com