
����Ŀ��������CuSO4��5H2O���й㷺����;��ij�о���ѧϰС������ij��ʵ����ϡ���ᡢϡ������Һ�Ʊ�������ʵ���������£�

��ش��������⣺
��1������XΪ___��___��
��2��NO��Ҫ�������ã�д��NO�������H2O��Ӧ��������Ļ�ѧ����ʽ___��
��3������48g��CuO��������Ϊ20%��ͭ�ۣ���һ������ϡ���ᡢϡ������Һǡ����ȫ��Ӧ����CuSO4������
�����������ɵ���������Ϊ___g��
��ԭ���Һ���������������ʵ���֮�ȡ�___��д��������̣�
���𰸡�����Ũ�� ��ȴ�ᾧ 4NO+3O2+2H2O=4HNO3 180g ͭ�����ᷴӦ�����ӷ���ʽ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
�����ĵ�����n��HNO3��=![]() 0.4mol��
0.4mol��
������Ԫ���غ�n��H2SO4��=n��CuSO4��5H2O��=0.72mol��
��n��H2SO4����n��HNO3��=0.72mol��0.4mol=9��5
��������
��1��ͭ����ϡ���ᡢϡ���ᷴӦ��������ͭ��Һ������Һ��������Ũ������ȴ�ᾧ�������õ����壻
��2��NO�������H2O��Ӧ�������ᣬ��ϵ�ʧ�����غ㡢�����غ���ƽ����ʽ��
��3������CuO��Cu�����������ͭԪ�������غ�ɼ���CuSO4��5H2O����������Ϸ�Ӧ�����ӷ���ʽ����ԭ���Һ���������������ʵ���֮�ȡ�
��1��ͭ����ϡ���ᡢϡ���ᷴӦ��������ͭ��Һ����õ�������Ӧ����Һ��������Ũ������ȴ�ᾧ������Ȼ���ˡ�ϴ�ӡ�����ɵõ����壻
��2��NO�������H2O��Ӧ�������ᣬ��Ӧ�ķ���ʽΪ4NO+3O2+2H2O=4HNO3��
��3����n��CuO��=![]() =0.12mol�� n��Cu��=
=0.12mol�� n��Cu��=![]() =0.6mol��
=0.6mol��
n��CuSO4��5H2O��=0.12mol+0.6mol=0.72mol��
m��CuSO4��5H2O��=0.72mol��250g/mol=180g�����������ɵ���������Ϊ180g��
��ͭ�����ᷴӦ�����ӷ���ʽ��3Cu+8H++2NO3-=3Cu2++2NO��+4H2O
�����ĵ�����n��HNO3��=![]() 0.4mol��
0.4mol��
������Ԫ���غ�n��H2SO4��=n��CuSO4��5H2O��=0.72mol��
��n��H2SO4����n��HNO3��=0.72mol��0.4mol=9��5��



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������й�������ԭ��Ӧ��������ȷ����( )
A.������ԭ��Ӧ����һ��Ԫ�ر�����ʱ��һ������һ��Ԫ�ر���ԭ
B.ij����Ԫ��M�ɻ���̬��Ϊ����̬��Mһ������ԭ
C.��˫���ű�ʾ���з�Ӧ�ĵ���ת��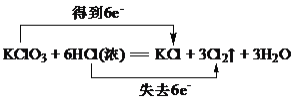
D.�ǽ��������ڷ�Ӧ��ֻ����������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�����Ȼ���������ýȾ����������ͼ��ʾװ�ÿ����Ʊ����Ȼ��������ּг�װ������ȥ��

�й���Ϣ���±���
��ѧʽ | SnCl2 | SnCl4 |
�۵�/�� | 246 | 33 |
�е�/�� | 652 | 144 |
�������� | ��ɫ���壬������ | ��ɫҺ�壬��ˮ�� |
�ش��������⣺
��1����װ��������A������Ϊ___________��
��2���ü�װ����������MnO4����ԭΪMn2+���÷�Ӧ�����ӷ���ʽΪ________________��
��3����װ����ͼ���Ӻã���������ԣ���������Ũ���ᣬ���۲쵽__________��������ʼ���ȶ�װ�ã����ۻ����ʵ����������������������ȶ�װ�ã���ʱ�������ȶ�װ�õ�Ŀ���ǣ��ٴٽ�����������Ӧ����_______________________��
��4����װ�õ�����____________�����ȱ����װ�ã����ܷ����ĸ���Ӧ�Ļ�ѧ����ʽΪ_________����װ�õ�������_____������ţ���
A����ֹ������CO2���������װ��
B����ȥδ��Ӧ����������ֹ��Ⱦ����
C����ֹˮ����������װ�õ��Թ���ʹ����ˮ��
D����ֹ������O2������װ�õ��Թ���ʹ��������
��5��ijͬѧ��Ϊ��װ���еķ�Ӧ���ܲ���SnCl2���ʣ������Լ��в������ڼ���Ƿ����SnCl2 ����_______������ţ���
A��H2O2��Һ B�����Ը��������Һ C��AgNO3��Һ D����ˮ
��6����Ӧ����ȥ����1.19 g����Ӧ������װ�õ��Թ����ռ���2.04 g SnCl4����SnCl4�IJ���Ϊ_______������2λ��Ч���֣���
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���������ӷ���ʽ����뷽��ʽ��ȷ����
A. NaHSO3��Һ�����ԣ�![]()
B. ��Na2SiO3��Һ��ͨ������CO2��![]()
C. ��ҵ��Ư�۵ķ�Ӧ��![]()
D. ��Na2S2O3��Һ�еμ�ϡ���![]()
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����NAΪ�����ӵ�������ֵ������˵����ȷ����
A. 22.4LCl2��������ˮ��������Һ��Cl2��Cl-��HClO��ClO-����������ΪNA
B. ��״���£�38g3H2O2�к���3NA���ۼ�
C. �����£���5.6g����Ͷ������Ũ�����У�ת��0.3NA����
D. 0.1molL-1MgCl2��Һ�к��е�Mg2+��Ŀһ��С��0.1NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���ڷ�Ӧ 3Cl2��6KOH![]() KClO3��5KCl��3H2O �У�����˵����ȷ���ǣ�( )
KClO3��5KCl��3H2O �У�����˵����ȷ���ǣ�( )
��Cl2����������KOH�ǻ�ԭ�� ��KCl�ǻ�ԭ���KClO3���������� �۷�Ӧ��ÿ����3 mol Cl2��������5mol ���ӷ���ת�� �ܱ������뱻��ԭ����ԭ�����ʵ���֮��Ϊ5��1
A.�٢�B.�ڢ�C.�ڢۢ�D.�ڢ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��ClO2��һ�����������������������Ƴ�NaClO2���壬�Ա���������棬�������ⷨ��NaClO2�����ʵ��װ����ͼ��ʾ��
��֪����2NaC1O3+H2O2+H2SO4=2C1O2��+O2��+Na2SO4+2H2O
2ClO2+H2O2+2NaOH=2NaClO2+O2��+2H2O
��ClO2�۵�-59�����е�11����Ũ�ȹ���ʱ�����ֽ⣻
��H2O2�е�150��
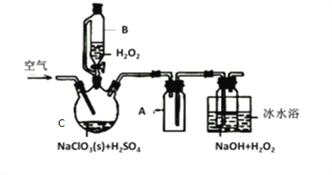
��1������C��������__________________������A��������_________________����ˮԡ��ȴ��Ŀ����_____________________��___________________________��
��2����װ�ò����Ƶķ�����________________________��
��3���������ٹ���������������NaClO2���ʣ��Խ�����ԭ�������ٹ���ʱ��___________���������ٹ���ʱ��________________��
��4��Cl-����ʱ���ClO2�����ɡ���Ӧ��ʼʱ��C�м����������ᣬClO2���������ʴ����ߣ����������������ù��̿��ܾ�������ɣ��뽫�䲹��������
��_____________________________�������ӷ���ʽ��ʾ��
��H2O2+Cl2=2Cl-+O2+2H+
��5��NaClO2���Ȳⶨ��
��ȷ��ȡ����NaClO2��Ʒ10.0g���ձ��У�������������ˮ�����ĵ⻯�ؾ��壬�ٵ���������ϡ���ᣬ��ַ�Ӧ��C1O2-�IJ���ΪCl-���������û��Һ���250mL������Һ��
��ȡ25.00mL����Һ����2.0mol��L-1Na2S2O3��Һ�ζ�(I2+2S2O32-=2I-+S4O62-)���Ե�����Һ��ָʾ�����ﵽ�ζ��յ�ʱ������Ϊ__________________________���ظ��ζ�3�Σ����Na2S2O3��Һƽ������Ϊ20.00mL�������Ʒ��NaClO2����������Ϊ_________________����M(NaClO2)=90.5g/mol��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������һ�ֶ�Ԫ���ᣬ��������ԭ�����������ȡ�ijУ����С���ͬѧ�������C2H2������ȡH2C2O4��2H2O���ش��������⣺
(1)�����ͬѧ�Ե�ʯ(��Ҫ�ɷ�CaC2������CaS��Ca3P2���ʵ�)Ϊԭ�ϣ�������ͼ1װ����ȡC2H2��

�ٵ�ʯ��ˮ��Ӧ�ܿ죬Ϊ�˼�����Ӧ���ʣ�װ��A�г��ñ���ʳ��ˮ����ˮ֮�⣬�����Բ�ȡ�Ĵ�ʩ��__________��дһ�ּ��ɣ���
��װ��B�У�NaClO��H2S��PH3 ����Ϊ���ἰ���ᣬ��������ԭΪNaCl������PH3�����������ӷ���ʽΪ______���ù����У����ܲ����µ���������Cl2����ԭ���ǣ� _____________�������ӷ���ʽ�ش𣩡�
(2)�����ͬѧ�����������ϣ���Hg(NO3)2��������Ũ��������C2H2��ȡH2C2O4��2H2O���Ʊ�װ������ͼ2��ʾ��
��װ��D�ж�����ݵ�������______________________��
��װ��D������H2C2O4�Ļ�ѧ����ʽΪ____________________________��
�۴�װ��D�еõ���Ʒ�����辭��_____________����������ƣ������ˡ�ϴ�Ӽ����
(3)��������˲ⶨ�����Ʒ��H2C2O4��2H2O����������ʵ�顣���ǵ�ʵ�鲽�����£�ȷ��ȡm g��Ʒ����ƿ�У���������������ˮ�ܽ⣬�ټ�������ϡ���ᣬȻ����c mol��L��1����KMnO4����Һ���еζ����յ㣬�����ı���ҺV mL��
�ٵζ��յ��������______________________��
�ڵζ������з�����ɫ���ʿ�ʼ�������ӿ죬�������ܵ�ԭ����_______________��
�۲�Ʒ��H2C2O4��2H2O����������Ϊ_______________���г��� m��c��V �ı���ʽ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ĺ���Ӱ��������ܡ�һ�ֲⶨ�����ķ����ǽ���������ת��Ϊ�����������壬���ò���װ�ý��вⶨ��ij�ⶨ�������������£�
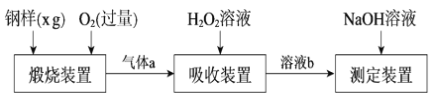
��1������a����Ҫ�ɷ���CO2��______��______��
��2��������������FeS����ʽ���ڣ�����װ���з����Ļ�ѧ��ӦΪ3FeS��5O2 ![]() 1______ ��3______��___________
1______ ��3______��___________
��3������װ���У�H2O2����SO2�Ļ�ѧ����ʽ��_________________��
��4����NaOH��Һ�к����ɵ���Һb������z mLNaOH��Һ��������1 mLNaOH��Һ�൱���������Ϊy g����ø����������������Ϊ______��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com