
|
��V1 mL��1.0 mol/L��HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼�¶ȣ�ʵ������ͼ��ʾ(ʵ����ʼ�ձ���V1��V2��50 mL)������˵����ȷ����
| |
| [����] | |
A�� |
����ʵ��ʱ�����¶�Ϊ22�� |
B�� |
ʵ������ڸ÷�Ӧ�л�ѧ��ת��Ϊ���� |
C�� |
NaOH��Һ��Ũ��ԼΪ1.0 mol/L |
D�� |
��ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ |
 ���ѵ����Ԫ��ĩ���100��ϵ�д�
���ѵ����Ԫ��ĩ���100��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ��������ʡĵ����һ��2010��2011ѧ��߶���ѧ�����п��Ի�ѧ�������� ���ͣ�013
|
��V1 mL��1.0 mol��L��1��HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ��������ͼ��ʾ(ʵ����ʼ�ձ���V1��V2��50 mL)������������ȷ����
| |
| [����] | |
A�� |
����ʵ��ʱ�����¶�Ϊ22�� |
B�� |
��ʵ�������ѧ�ܿ�ת��Ϊ���� |
C�� |
NaOH��Һ�����ʵ���Ũ��ԼΪ1.0 mol/L |
D�� |
��ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��ɽ��ʡ�����н������2011��2012ѧ��߶���ѧ�����п��Ի�ѧ���� ���ͣ�022
ij�о�С�齫V1 mL��1.0 mol/L��HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ��������ͼ��ʾ(ʵ����ʼ�ձ���V1��V2��50 mL)���ش��������⣺
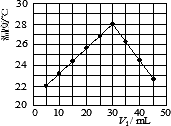
(1)�о�С������ʵ��ʱ�����¶�________(����ڡ��������ڡ����ڡ�)22�森
(2)����ɼ�ͼ�ο�֪��V1��V2��________ʱ�����ǡ����ȫ�кͣ��˷�Ӧ����NaOH��Һ��Ũ��ӦΪ________mol/L��
(3)ʵ��ʱ������ڼ������ȼ��л�ϣ�����________���裬ʹ��Һ��Ͼ��ȣ����йؼ���֪�˷�Ӧ���ų�Q kJ����������д���˷�Ӧ���Ȼ�ѧ����ʽ��________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ�����а��˸���2010��2011ѧ��߶���ѧ����ĩ���Ի�ѧ����(ѡ��) ���ͣ�058
50 ml��1.0 mol��L��1������50 mL��1.1 mol��L��1��NaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ�����зų������������к��ȣ��ش��������⣺
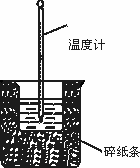
�ٴ�ʵ��װ���Ͽ������ձ���û��________���ᵼ����õ��к�����ֵ________(�ƫ��ƫС������Ӱ�족)��ʵ��װ����Ҳȱ�ٻ��β�������������û���ͭ�ʽ�������棬�ᵼ����õ��к�����ֵ________(�ƫ��ƫС������Ӱ�족)��
����ʵ���зֶ�ΰ�NaOH��Һ����ʢ�������С�ձ��У�����õ��к�����ֵ
(�ƫ��ƫС������Ӱ�족)
����ʵ���и���60 mL��1.0 mol��L��1�����50 mL��1.1 mol��L��1��NaOH��Һ���з�Ӧ��������ʵ����ȣ����ų�������________(���ȡ�����ȡ�)�������к���________(���ȡ�����ȡ�)����ʵ����NaOH��Һ��Ũ�ȴ�������Ũ�ȵ�������________��
��������ͬŨ�Ⱥ�����İ�ˮ����NaOH��Һ��������ʵ�飬��õ��к�����ֵ��________(�ƫ��ƫС������Ӱ�족)��
�ݵ����µ���10��ʱ����ʵ�飬��ʵ��������ɽϴ�����ԭ����________��
������V2 mLδ֪Ũ�ȵ�NaOH��Һ��������ʢ��V1 mL��1.0 mol/L��HCl��Һ��С�ձ��л�Ͼ��Ⱥ��������¼��Һ�¶ȣ���ÿ��ʵ���о�����V1��V2��10.0 mL��ʵ������ͼ��ʾ(���¶�Ϊ�����꣬V1Ϊ������)������ʵ��ʱ�����¶�________22��(���������)��NaOH��Һ��Ũ��ԼΪ________mol/L��
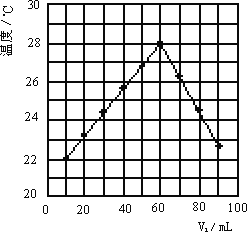
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ʡ��������2011��2012ѧ���һ��ѧ�����п��Ի�ѧ���� ���ͣ�058
��һ��Ũ�ȵ�ϡ������ϡNaOH��Һ����ͼ��ʾ��װ���н����кͷ�Ӧ��ͨ���ⶨ��Ӧ���������ų��������ɼ����к��ȣ���֪ǿ����ǿ����к���Ϊ57.3 KJ/mol��ˮ�ı�����Ϊ4.2 J/(g����)���й�����������ݵļ��㹫ʽ��Q��m��c����t(QΪ������mΪ���ʵ�������cΪ�����ݣ���t�����¶ȵı仯ֵ)
�ش����������
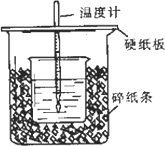
(1)��ʵ��װ���Ͽ���ͼ����ȱ�ٵ�һ�ֲ�����Ʒ�ǡ�________
(2)��V1 mL��1.0 mol/L��HCl��Һ��V2 mLδ֪Ũ�ȵ�NaOH��Һ��Ͼ��Ⱥ��������¼��Һ�¶ȣ�ʵ������ͼ��ʾ(ʵ����ʼ�ձ���V1��V2��50 mL)��
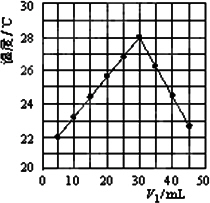
����������ȷ����________
A������ʵ��ʱ�����¶�Ϊ22��
B����ʵ�������ѧ�ܿ���ת��Ϊ����
C��NaOH��Һ��Ũ��Լ��1.00 mol/L
D����ʵ�������ˮ���ɵķ�Ӧ���Ƿ��ȷ�Ӧ
(3)V1��30 mLʱHCl��NaOHǡ�÷�Ӧ������������������������¶ȱ仯ֵԼΪ________(��������������ʱ�ɽ�ϡ��Һ���ܶȼ������ݿ�����ˮ������ͬ������Һ���ʱ����仯���Բ���)����������ͼ���¶ȱ仯�ɶ����¶ȱ仯ֵԼ7�棻�������������ƫ�ͣ������������ԭ���С�________
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com