
����Ŀ�������ũҵ�����벻�����ʣ��������еĵ��ʶ���Ϊԭ�ϣ�ij��ѧ��ȤС��������ͼװ���Ʊ�������̽��������ʡ�

��1��װ��A �У�ʢ��Ũ��ˮ����������Ϊ_____��װ��B ��������_____��
��2�����Ӻ�װ�ò�����װ�õ������Ժ�װ��ҩƷ��Ȼ��Ӧ��_____���� I ���
�����������Բ����ƿ�м��백ˮ ����װ��C
��3��ʵ���й۲쵽C �� CuO ��ĩ��죬D ����ˮ����ͭ���������ռ���һ�ֵ������壬��÷�Ӧ��ػ�ѧ����ʽΪ_____���÷�Ӧ֤���������л�ԭ�ԣ������������ڴ��������µķ�ӦҲ��������һ���ʣ��÷�Ӧ��ѧ����ʽΪ_____��
��4����ʵ��ȱ��β������װ�ã���ͼ������������β����װ����_____����װ����ţ���

��5��ʵ���һ�������ͼ��ʾװ���Ʊ���������ѧ��Ӧ����ʽΪ_____��

��6���ֽ� 1.92gCu Ͷ�뵽һ������ŨHNO3 �У�Cu ��ȫ�ܽ⣬����������ɫԽ��Խdz�����ռ�����״���� 672mL ��NOX ������壬��ʢ�д����������������ˮ���У�ͨ���״����һ�������O2��ǡ��ʹ������ȫ����ˮ����ͨ���״���µ� O2 �����Ϊ_____��
���𰸡���Һ©�� ���ﰱ�� I 3CuO +2NH3 ![]() 3Cu + N2 + 3H2O 4NH3+5O2
3Cu + N2 + 3H2O 4NH3+5O2![]() 4NO + 6H2O II��III Ca(OH)2 + 2NH4Cl
4NO + 6H2O II��III Ca(OH)2 + 2NH4Cl![]() CaCl2 + 2NH3��+2H2O 0.336L
CaCl2 + 2NH3��+2H2O 0.336L
��������
Ũ��ˮ�ڼ�ʯ�һ���ʯ�ҵ������»ӷ����ɰ�������B���ü�ʯ�Ҹ����C�м��Ȱ���������ͭ����������ԭ��Ӧ����ˮ��������ͭ��D�����ڼ����Ƿ�����ˮ���������ˮ���գ�������������ˮ��ע���ֹ������
��1������ͼ��װ�ã�װ��A ��ʢ��Ũ��ˮ����������Ϊ��Һ©����װ��B�Ǽ�ʯ�ң���ʯ�Ҳ��백����Ӧ����������Ǹ��ﰱ�����ʴ�Ϊ����Һ©�������ﰱ����
��2�����Ӻ�װ�ò�����װ�õ������Ժ�װ��ҩƷ��Ȼ��Ӧ�ȴ����������Բ����ƿ�м��백ˮ���ټ���C���ƾ��ƣ��ʴ�Ϊ��I��
��3��ʵ���й۲쵽C��CuO��ĩ��죬D����ˮ����ͭ���������ռ���һ�ֵ������壬�����е����ϼ����ߣ�����ͭ��ͭ���ϼ۽��ͣ�����Ԫ���γ�ˮ����÷�Ӧ��ѧ����ʽΪ3CuO +2NH3 ![]() 3Cu + N2 + 3H2O���÷�Ӧ֤���������л�ԭ�ԣ������������ڴ��������µķ�ӦҲ��������һ���ʣ��÷�Ӧ��ѧ����ʽΪ4NH3+5O2
3Cu + N2 + 3H2O���÷�Ӧ֤���������л�ԭ�ԣ������������ڴ��������µķ�ӦҲ��������һ���ʣ��÷�Ӧ��ѧ����ʽΪ4NH3+5O2![]() 4NO + 6H2O���ʴ�Ϊ��3CuO +2NH3
4NO + 6H2O���ʴ�Ϊ��3CuO +2NH3 ![]() 3Cu + N2 + 3H2O��4NH3+5O2
3Cu + N2 + 3H2O��4NH3+5O2![]() 4NO + 6H2O��
4NO + 6H2O��
��4����ʵ��ȱ��β������װ�ã�������������ˮ��Ҫע���������II��III���ܷ��������ʴ�Ϊ��II��III��
��5��ʵ���һ����Ȼ�狀��������Ƽ��ȷ�Ӧ�����Ȼ��ƺͰ������仯ѧ��Ӧ����ʽΪCa(OH)2 + 2NH4Cl![]() CaCl2 + 2NH3��+2H2O���ʴ�Ϊ��Ca(OH)2 + 2NH4Cl
CaCl2 + 2NH3��+2H2O���ʴ�Ϊ��Ca(OH)2 + 2NH4Cl![]() CaCl2 + 2NH3��+2H2O��
CaCl2 + 2NH3��+2H2O��
��6������������ͭʧȥ���ӵ����ʵ������������Ϊ��������õ����ӵ����ʵ��������������Ϊ����ʧȥ���ӵ����ʵ����������õ����ӵ����ʵ�����ȣ���ͭʧȥ���ӵ����ʵ������������õ����ӵ����ʵ�����4n(O2)=2n(Cu)��4n(O2)=2��![]() ��n(O2)=0.015mol����ͨ���״���µ� O2 �����ΪV = nVm = 0.015mol�� 22.4 L��mol1 =0.336L���ʴ�Ϊ��0.336L��
��n(O2)=0.015mol����ͨ���״���µ� O2 �����ΪV = nVm = 0.015mol�� 22.4 L��mol1 =0.336L���ʴ�Ϊ��0.336L��


 ��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
��˼ά������ҵ���ټ��ִ�ѧ������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ���״����ӽ���Ĥȼ�ϵ���н��״�����ת��Ϊ���������ַ�Ӧԭ����
�� CH3OH(g)��H2O(g)===CO2(g)��3H2(g)����H����49.0 kJ/mol
�� CH3OH(g)��![]() O2(g)===CO2(g)��2H2(g)����H����192.9 kJ/mol
O2(g)===CO2(g)��2H2(g)����H����192.9 kJ/mol
����˵����ȷ���� �� ��
A. CH3OH��ȼ����Ϊ192.9 kJ/mol
B. ��Ӧ���е������仯��ͼ��ʾ
C. CH3OHת���H2�Ĺ���һ��Ҫ��������
D. ��������֪��Ӧ��CH3OH(l)��![]() O2(g)===CO2(g)��2H2(g)����H>��192.9 kJ/mol
O2(g)===CO2(g)��2H2(g)����H>��192.9 kJ/mol
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����5.2g����̬��Ȳ��C2H2����������ȼ�գ����ɶ�����̼��Һ̬ˮ���ų�260kJ�����������Ȼ�ѧ����ʽΪ��___________________________________________________________����֪H2O��l��==H2O��g����H= +44kJ/mol����11.2L����״������Ȳ��ȫȼ��������̬ˮʱ�ų���������_________kJ��
����֪��CH4 ��ȼ����Ϊ890 kJ/mol��H2����ֵΪ142.5kJg-1�����б�״����22.4 L CH4��H2�Ļ��������ȫȼ��ʱ���ų�������Ϊ527kJ������������CH4 ��H2�������_____________
�۽�1mol NO2Ͷ�뵽1L�����н��� 2NO2(g)![]() N2O4(g)��Ӧ���ﵽƽ������ѹ����ԭ����һ���ٴδﵽƽ�⣬���һ��ƽ����ϵ����ɫ�ȵڶ���ƽ�����ɫ_____________
N2O4(g)��Ӧ���ﵽƽ������ѹ����ԭ����һ���ٴδﵽƽ�⣬���һ��ƽ����ϵ����ɫ�ȵڶ���ƽ�����ɫ_____________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��Ϊ̽������A����ɺ����ʣ����ʵ�鲢�������ת����

��֪��X�����ֻ�������ɣ�����Xͨ��Ʒ����Һ����Һ��ɫ������Xͨ������˫��ˮ�У�X��ȫ����������ֻ�õ�һ��ǿ�ᣬ��ϡ�͵�1000mL�������Һ��PH=1������Һ2�еμ�KSCN��Һ����Һ��Ѫ��ɫ����ش�
��1������A�Ļ�ѧʽ______________��
��2��д����Ӧ�ٵĻ�ѧ����ʽ____________��
��3��д����Ӧ��������A�����ӷ���ʽ______________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������Զ�������г�Ӧ�ø���ԭ���ԭ����ƵĴ���������ͼΪ��صĹ���ʾ��ͼ:������ɢ���봫�����������е缫�Ϸ�����Ӧ���������ͻ���յ����źš��±��г��˴������弰���е缫�ϲ��ַ�Ӧ���


������˵������ȷ����
A. ����������ʱ�����е缫������ظ���
B. ��� Cl2 ����ʱ,���е缫�ĵ缫��ӦʽΪ Cl2+2e����2Cl��
C. ��� H2S ����ʱ���Ե缫����������Ե缫�ϵĵ缫��ӦʽΪ O2��2H2O��4e����4OH��
D. ��� Cl2 �� CO ���������ͬ�����ݿ�������ʱ���������ϵ�����С��������ͬ
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ�������ӵ�������ֵΪNA������˵������ȷ����
A.���³�ѹ�£�2 g D2O �к�������ΪNA
B.1mol����ϩ��![]() �������к���̼̼˫���ĸ���Ϊ4 NA
�������к���̼̼˫���ĸ���Ϊ4 NA
C.0.5mol�ǻ�������������Ϊ4.5NA
D.28g��ϩ�ͱ�ϩ��C3H6���Ļ�����庬�е�̼ԭ����ĿΪ2NA
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ��������ͼ��ʾ����װ�D���һ����ⱥ��ʳ��ˮ�����ⶨ������������ ����ͼ������������Ե�ʵ��װ�á�

��1����ѡ��������ʱ�����ӿڵ�˳���ǣ�����ӿڵĴ�����ĸ����a�� �� �� ��b�� �� �� ��______________
��2��֤����Cl2���ɵ�ʵ��������____________��
��3��ʵ��ʱ��װ���е����缫�ӵ�Դ��____________����ʯī�缫�ķ�ӦʽΪ____________��
��4��װ��E��ȡ�������ʱ��Ӧ���е�ʵ�������____________����ʵ�������װ��E�Ķ�����������ɱ�״����Ϊ5.60mL��������Һ�����ǡ��Ϊ50.0mL������Һ��OH-��Ũ��Ϊ________mol��L-1��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ����ҵ�кܶ���Ҫ�Ļ���ԭ�϶���Դ��ʯ�ͻ�������ͼ�еı�����ϩ���л���A�ȣ�����A�IJ���������������һ�����ҵ�ʯ�ͻ�����չˮƽ����ش��������⣺

(1)A�Ľṹ��ʽΪ________________����ϩ���й����ŵ�����Ϊ__________________________________________
(2)д�����з�Ӧ�ķ�Ӧ������___________________����________________________
(3)����˵����ȷ������________��
A.�������л���ŨHNO3��H2SO4�����䵹�뵽NaOH��Һ�У����ã���Һ
B.��ȥ���������е����ᣬ��NaOH��Һ����Һ
C.�۱�ϩ���ܹ�ʹ���Ը��������Һ��ɫ
D.�л���C���ϩ������ͬϵ��
(4)д�����з�Ӧ����ʽ��
��B��CH3CHO_________________________________________
�ܱ�ϩ�� + B����ϩ������_____________________
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����Ŀ������ͼ��ʯī���缫�ĵ����У�����500mL���������ʵ�ij��ɫ��������Һ���е�⣬�۲쵽A�缫�����к�ɫ�Ĺ�̬�������ɣ�B�缫����ɫ�������ɡ���ش��������⣺
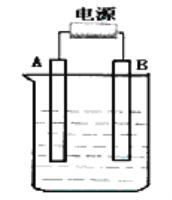
��1��д�����ʱ��Ӧ�������ӷ���ʽ____________________��
��2��������Һ�е�ԭ��������ȫ����ֹͣ��⣬ȡ��A�缫��ϴ�ӡ�����������缫����6g��������Һ��pHΪ_____________��Ҫʹ������Һ�ָ������ǰ��״̬���������_____________��������Ϊ_____________g����������ǰ����Һ��������䣩
��3����ԭ��ҺΪ1L K2SO4��CuSO4�Ļ����Һ����c ��SO42-) =2.0mol/L����ͼװ�õ�⣬���������ռ���22.4L���壨��״����ʱ��ֹͣ��⡣��ԭ��Һ�е�c(K+)=_____________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com