

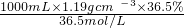 =11.9mol��
=11.9mol��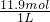 =11.9mol/L���ʴ�Ϊ��11.9��
=11.9mol/L���ʴ�Ϊ��11.9�� ������ʵ���Ũ�ȣ��ڸ��ݸ���ʽ�Ƿ�������й��жϣ�
������ʵ���Ũ�ȣ��ڸ��ݸ���ʽ�Ƿ�������й��жϣ� �������������ʵ����ʵ��������Һ�������Ӱ���жϣ�
�������������ʵ����ʵ��������Һ�������Ӱ���жϣ�

 ��У����ϵ�д�
��У����ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
 ��2011?��������ģ���̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�
��2011?��������ģ���̶�������CO2������Ч��������Դ�������ٿ����е��������壮��ҵ�������о�����CO2�������״�ȼ�ϵķ������÷����Ļ�ѧ����ʽ�ǣ�| [CH3OH]?[H2O] |
| [CO2]?[H2]3 |
| [CH3OH]?[H2O] |
| [CO2]?[H2]3 |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
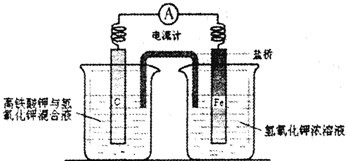
��������ͭ��ͭ����ɵĻ���ijͬѧ������ͼ��ʾװ�ã�ͨ���ⶨ�����������ʵ��ǰ��U�������仯��ȷ�������������ͭ������������
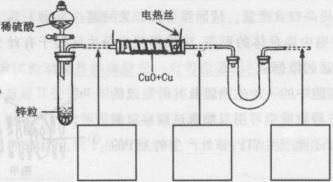 |
�ش��������⣺
(1)U�ι��п��Լ���������� (�����)��
A��Ũ![]() B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
B����ˮ����ͭ��ĩ C����ˮ�Ȼ��ƿ���
(2)���в��谴ʵ�����˳��ӦΪ (����ĸ)��
a��ֹͣͨ������b������˿ͨ�磻c��ͨ��������d��װ�������Լ�飻e������˿ֹͣͨ�硣
| ʵ��Ŀ�ģ����� ʵ��ԭ�������� ʵ��������ҩƷ������ ʵ��װ�ã����� ʵ�����ݴ��������� ʵ�������������� ʵ���������ۣ����� |
(3)Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�
(4)ʵ�������ͬѧ������ʦ��ʵ�鱨����Ҫ��
Ŀ��ͼ(������������)���������ʵ�鱨���дҪ��
�Դ˷ݱ����������ۣ�������������������д��������������������Ŀո���д����ȱ��Ŀ ��
(5)��ʦ����ʵ�鱨���ָ�����ı�ʵ��ԭ��������
�Ƴ����Ӽ���ʵ�鷽�������û�ѧ����ʽ��ʾ�����
���·����ķ�Ӧԭ�� ���÷�����ⶨ������ ��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
��������ͭ��ͭ����ɵĻ���ijͬѧ������ͼ��ʾװ�ã�ͨ���ⶨ�����������ʵ��ǰ��U�������仯��ȷ�������������ͭ������������
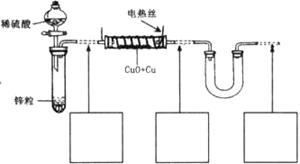
�ش��������⣺
��1��U�ι��п��Լ����������_________������ţ���
A��Ũ![]() B����ˮ����ͭ�� C����ˮ�Ȼ��ƿ���
B����ˮ����ͭ�� C����ˮ�Ȼ��ƿ���
��2�����в��谴ʵ�����˳��ӦΪ_________������ĸ����
A��ֹͣͨ������ B������˿ͨ�磻 C��ͨ��������
D��װ�������Լ�飻 E������˿ֹͣͨ�硣
 ��3��Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�
��3��Ϊȷ�ⶨ���ݣ�����Ϊ��װ���Ƿ�����?����Ҫ�Ľ�������ͼ����������ķ����ڻ����������ӵ�װ��ʾ��ͼ��ע����Ҫ���������ơ�������Ľ�����װ��ͼ�����߲��ָ�Ϊʵ�ߣ�
��4��ʵ�������ͬѧ������ʦ��ʵ�鱨����Ҫ��
Ŀ��ͼ�������������ԣ����������ʵ�鱨���
дҪ�Դ˷ݱ����������ۣ���������������
����д��������������������Ŀո���д����ȱ
��Ŀ______ _______ __��
��5����ʦ����ʵ�鱨���ָ�����ı�ʵ��ԭ��������
�Ƴ����Ӽ���ʵ�鷽�������û�ѧ����ʽ��ʾ
����Ƶ��·����ķ�Ӧԭ��____ ________��
�÷�����ⶨ������____________________��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com