
ŅŃÖŖA”¢B”¢C”¢D”¢E”¢FŹĒŗ¬ÓŠĶ¬Ņ»ÖÖŌŖĖŲµÄ»ÆŗĻĪļ£¬ĘäÖŠFŹĒÄÜŹ¹ŗģÉ«ŹŖČóŹÆČļŹŌÖ½±äĄ¶µÄĘųĢ壬ĖüĆĒÖ®¼äÄÜ·¢ÉśČēĻĀ·“Ó¦
¢ŁA£«H2O”śB£«C””¢ŚC£«F”śD
¢ŪD£«NaOH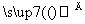 F£«E£«H2O
F£«E£«H2O
(1)Š“³öĖüĆĒµÄ»ÆѧŹ½£ŗC__________”¢D__________”¢E________”£
(2)Š“³öø÷²½·“Ó¦µÄĄė×Ó·½³ĢŹ½
¢Ł________________________________________________________________________£¬
¢Ū________________________________________________________________________”£
(3)¹¤ŅµÉś²śCµÄ¹ż³ĢÖŠÓŠČēĻĀŅ»²½·“Ó¦£¬¼“F¾“ß»ÆŃõ»ÆÉś³ÉBŗĶH2O£¬Š“³öøĆ²½·“Ó¦µÄ»Æѧ·½³ĢŹ½
________________________________________________________________________ӣ

| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĻĀ±ķø÷×éĪļÖŹÖŠ£¬ĪļÖŹÖ®¼äĶعżŅ»²½·“Ó¦¾ĶÄÜŹµĻÖČēĶ¼ĖłŹ¾×Ŗ»ÆµÄŹĒ(””””)
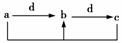
| ””””ĪļÖŹ Ń”Ļī”””” | a | b | c | d |
| A | Al | Al(OH)3 | NaAlO2 | NaOH |
| B | CH3CH2OH | CH3CHO | CH3COOH | O2 |
| C | Na2CO3 | NaHCO3 | NaOH | CO2 |
| D | Cl2 | FeCl3 | FeCl2 | Fe |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ijĪŽÉ«ČÜŅŗX£¬ÓÉNa+”¢Ag+”¢Ba2+”¢Al3+”¢AlO2﹣”¢MnO4﹣”¢CO32﹣”¢SO42﹣ÖŠµÄČōøÉÖÖĄė×Ó×é³É£¬Č”ČÜŅŗ½ųŠŠČēĶ¼ŹµŃé£ŗ
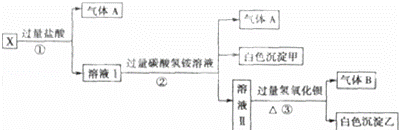
£Ø1£©°×É«³Įµķ¼×ŹĒ””””£®
£Ø2£©XČÜŅŗÖŠŅ»¶Ø“ęŌŚµÄĄė×ÓŹĒ””””£®
£Ø3£©°×É«³ĮµķŅŅÖŠŅ»¶ØÓŠ£ŗ””””£¬æÉÄÜÓŠ””””£®
£Ø4£©Čō½«¹żĮæµÄĘųĢåAÓėŹŹĮæµÄĘųĢåBĶØČėĖ®ÖŠ£¬Š“³ö·“Ó¦µÄ»Æѧ·½³ĢŹ½””3””£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ŗ¬HŌ×ÓøöŹżĪŖ1.806”Į1023øöNH3£¬ĘäÖŹĮæĪŖ(””””)
A£®17æĖ B£®0.17æĖ
C£®1.7æĖ D£®5.4æĖ
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ĪŖĮĖ¼ģŃéij¹ĢĢåĪļÖŹÖŠŹĒ·ńŗ¬ÓŠNH £¬ĻĀĮŠŹŌÖ½ŗĶŹŌ¼ĮŅ»¶ØÓĆ²»µ½µÄŹĒ(””””)
£¬ĻĀĮŠŹŌÖ½ŗĶŹŌ¼ĮŅ»¶ØÓĆ²»µ½µÄŹĒ(””””)
¢ŁÕōĮóĖ®””¢ŚNaOHČÜŅŗ””¢ŪŗģÉ«ŹÆČļŹŌÖ½””¢ÜĄ¶É«ŹÆČļŹŌÖ½””¢ŻĻ”ĮņĖį
A£®¢Ł¢Ż B£®¢Ü¢Ż
C£®¢Ł¢Ū D£®¢Ł¢Ü¢Ż
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
T ”ꏱ£¬½«6 mol CO2ŗĶ8 mol H2³äČė2 LĆܱÕČŻĘ÷ÖŠ£¬·¢Éś·“Ó¦CO2£Øg£©£«H2£Øg£©  CH3OH£Øg£©£«H2O£Øg£©£¬ČŻĘ÷ÖŠH2µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆČēĶ¼ÖŠŹµĻßĖłŹ¾”£Ķ¼ÖŠŠéĻß±ķŹ¾½öøıäijŅ»·“Ó¦Ģõ¼žŹ±£¬H2µÄĪļÖŹµÄĮæĖꏱ¼äµÄ±ä»Æ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
CH3OH£Øg£©£«H2O£Øg£©£¬ČŻĘ÷ÖŠH2µÄĪļÖŹµÄĮæĖꏱ¼ä±ä»ÆČēĶ¼ÖŠŹµĻßĖłŹ¾”£Ķ¼ÖŠŠéĻß±ķŹ¾½öøıäijŅ»·“Ó¦Ģõ¼žŹ±£¬H2µÄĪļÖŹµÄĮæĖꏱ¼äµÄ±ä»Æ”£ĻĀĮŠĖµ·ØÕżČ·µÄŹĒ
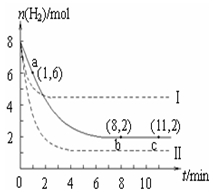
A£®·“Ó¦æŖŹ¼ÖĮaµćŹ±v£ØH2£©£½1 mol”¤L-1”¤min-1
B£®ČōĒśĻߢń¶ŌÓ¦µÄĢõ¼žøıäŹĒÉżĪĀ£¬ŌņøĆ·“Ó¦DH£¾0
C£®ĒśĻߢņ¶ŌÓ¦µÄĢõ¼žøıäŹĒ½µµĶŃ¹Ēæ
D£®T ”ꏱ£¬øĆ·“Ó¦µÄ»ÆŃ§Ę½ŗā³£ŹżĪŖ0£®125
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
I£®½«Ņ»¶ØĮæNO2ŗĶN2O4µÄ»ģŗĻĘųĢåĶØČėĢå»żĪŖ1L µÄŗćĪĀĆܱÕČŻĘ÷ÖŠ£¬ø÷Īļ
µÄŗćĪĀĆܱÕČŻĘ÷ÖŠ£¬ø÷Īļ
ÖŹÅضČĖꏱ¼ä±ä»ÆµÄ¹ŲĻµČēĶ¼1ĖłŹ¾”£
Ēė»Ų“š£ŗ
£Ø1£©ĻĀĮŠŃ”ĻīÖŠ²»ÄÜĖµĆ÷øĆ·“Ó¦ŅŃ“ļµ½Ę½ŗāדĢ¬µÄŹĒ £ØĢīŃ”Ļī×ÖÄø£©”£
A£®ČŻĘ÷ÄŚ»ģŗĻĘųĢåµÄŃ¹Ēæ²»Ėꏱ¼ä±ä»Æ¶ųøıä
B£®ČŻĘ÷ÄŚ»ģŗĻĘųĢåµÄĆÜ¶Č²»Ėꏱ¼ä±ä»Æ¶ųøıä
C£®ČŻĘ÷ÄŚ»ģŗĻĘųĢåµÄŃÕÉ«²»Ėꏱ¼ä±ä»Æ¶ųøıä
D£®ČŻĘ÷ÄŚ»ģŗĻĘųĢåµÄĘ½¾łĻą¶Ō·Ö×ÓÖŹĮæ²»Ėꏱ¼ä±ä»Æ¶ųøıä
£Ø2£©·“Ó¦½ųŠŠµ½10 minŹ±£¬¹²ĪüŹÕČČĮæ11£®38 kJ£¬ŌņøĆ·“Ó¦µÄČČ»Æѧ·½³ĢŹ½ĪŖ
£»
£Ø3£©¼ĘĖćøĆ·“Ó¦µÄĘ½ŗā³£ŹżK= ”£
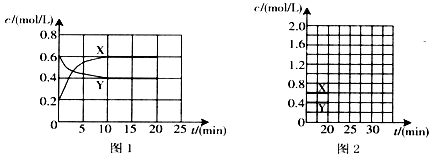
£Ø4£©·“Ó¦½ųŠŠµ½20 minŹ±£¬ŌŁĻņČŻĘ÷ÄŚ³äČėŅ»¶ØĮæNO2£¬10minŗó“ļµ½ŠĀµÄĘ½ŗā£¬“ĖŹ±²āµĆc£ØNO2£©=0£®9 mol£ÆL”£
¢ŁµŚŅ»“ĪĘ½ŗāŹ±»ģŗĻĘųĢåÖŠNO2µÄĢå»ż·ÖŹżĪŖw1£¬“ļµ½ŠĀĘ½ŗāŗó»ģŗĻĘųĢåÖŠNO2µÄĢå»ż·ÖŹżĪŖw2£¬Ōņw1 w2 £ØĢī”°>”±”¢”°=”±»ņ”°<”±£©£»
¢ŚĒėŌŚĶ¼2ÖŠ»³ö20 minŗóø÷ĪļÖŹµÄÅضČĖꏱ¼ä±ä»ÆµÄĒśĻߣØĒśĻßÉĻ±ŲŠė±ź³ö”°X”±ŗĶ”°Y”±£©”£
II£®£Ø1£©ŗ£Ė®ÖŠļ®ŌŖĖŲ“¢Įæ·Ē³£·įø»£¬“Óŗ£Ė®ÖŠĢįČ”ļ®µÄŃŠ¾æ¼«¾ßĒ±Į¦”£ļ®ŹĒÖĘŌģ»ÆѧµēŌ“µÄÖŲŅŖŌĮĻ”£ČēLiFePO4µē³Ų֊ijµē¼«µÄ¹¤×÷ŌĄķČēĻĀĶ¼ĖłŹ¾£ŗ
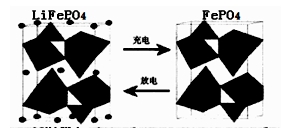
øƵē³ŲµÄµē½āÖŹĪŖÄÜ“«µ¼Li+µÄ¹ĢĢå²ÄĮĻ”£·ÅµēŹ±øƵē¼«ŹĒµē³ŲµÄ ¼«£ØĢī”°Õż”±»ņ”°øŗ”±£©£¬øƵē¼«·“Ó¦Ź½ĪŖ ”£
£Ø2£©ÓĆ“Ėµē³Ųµē½āŗ¬ÓŠ0£®1 mol/L CuSO4ŗĶ0£®1 mol/L NaClµÄ»ģŗĻČÜŅŗ100 mL£¬¼ŁČēµēĀ·ÖŠ×ŖŅĘĮĖ0£®02 mol e££¬ ĒŅµē½ā³ŲµÄµē¼«¾łĪŖ¶čŠŌµē¼«£¬Ńō¼«²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żŹĒ__________L£®
ĒŅµē½ā³ŲµÄµē¼«¾łĪŖ¶čŠŌµē¼«£¬Ńō¼«²śÉśµÄĘųĢåŌŚ±ź×¼×“æöĻĀµÄĢå»żŹĒ__________L£®
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
¼ģŃéäåŅŅĶéÖŠŗ¬ÓŠäåŌŖĖŲ“ęŌŚµÄŹµŃé²½Öč”¢²Ł×÷ŗĶĖ³ŠņÕżČ·µÄŹĒ( )
¢Ł¼ÓČėAgNO3ČÜŅŗ£»¢Ś¼ÓČėNaOHČÜŅŗ£»¢Ū¼ÓČėŹŹĮæHNO3£»
¢Ü¼ÓČČÖó·ŠŅ»¶ĪŹ±¼ä£»¢ŻĄäČ“”£
A.¢Ś¢Ü¢Ż¢Ū¢Ł B.¢Ł¢Ś¢Ü C.¢Ś¢Ü¢Ł D.¢Ś¢Ü¢Ż¢Ł
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗ
ČēĶ¼¼×”¢ŅŅŹĒµē»ÆѧŹµŃé×°ÖĆ”£
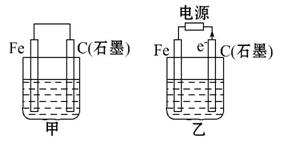
(1)Čō¼×”¢ŅŅĮ½ÉÕ±ÖŠ¾łŹ¢ÓŠ±„ŗĶNaClČÜŅŗ”£
¢Ł¼×ÖŠŹÆÄ«°ōÉĻµÄµē¼«·“Ó¦Ź½ĪŖ ””;
¢ŚŅŅÖŠ×Ü·“Ó¦µÄĄė×Ó·½³ĢŹ½ĪŖ ””;
¢Ū½«ŹŖČóµÄµķ·ŪKIŹŌÖ½·ÅŌŚŅŅÉÕ±ÉĻ·½,·¢ĻÖŹŌÖ½Ļȱ䥶ŗóĶŹÉ«,ÕāŹĒŅņĪŖ¹żĮæµÄCl2Ńõ»ÆĮĖÉś³ÉµÄI2”£Čō·“Ó¦ÖŠCl2ŗĶI2µÄĪļÖŹµÄĮæÖ®±ČĪŖ5”Ć1,ĒŅÉś³ÉĮ½ÖÖĖį,øĆ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ ””;
(2)Čō¼×”¢ŅŅĮ½ÉÕ±ÖŠ¾łŹ¢ÓŠCuSO4ČÜŅŗ”£
¢Ł¼×ÖŠĢś°ōÉĻµÄµē¼«·“Ó¦Ź½ĪŖ ””;
¢ŚČē¹ūĘšŹ¼Ź±ŅŅÖŠŹ¢ÓŠ200 mL pH = 5µÄCuSO4ČÜŅŗ(25”ę),Ņ»¶ĪŹ±¼äŗóČÜŅŗµÄpH±äĪŖ1,ČōŅŖŹ¹ČÜŅŗ»Öø“µ½µē½āĒ°µÄדĢ¬,æÉĻņČÜŅŗÖŠ¼ÓČė””””””””(ĢīŠ“ĪļÖŹµÄ»ÆѧŹ½)””””””””g”£
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com