
£Ø12·Ö£©Ķź³ÉŅŌĻĀŹµŃé£ŗ¢ŁÓĆÓŅĶ¼ĖłŹ¾µÄ×°ÖĆÖĘČ”äåŅŅĶ飻¢Ś½ųŠŠäåŅŅĶéµÄŠŌÖŹŹµŃ锣ŌŚŹŌ¹ÜIÖŠŅĄ“Ī¼ÓČė2 mL ÕōĮóĖ®”¢4 mLÅØĮņĖį”¢2 mL 95£„µÄŅŅ“¼ŗĶ3gäå»ÆÄĘ·ŪÄ©£¬ŌŚŹŌ¹Ü¢ņ֊עČėÕōĮóĖ®£¬ŌŚÉձ֊עČė×ŌĄ“Ė®”£¼ÓČČŹŌ¹ÜIÖĮĪ¢·Š×“Ģ¬Źż·ÖÖÓŗó£¬ĄäČ“”£ŹŌ»Ų“šĻĀĮŠĪŹĢā£ŗ
£Ø1£©ŹŌ¹ÜIÖŠÅØĮņĖįÓėäå»ÆÄĘ¼ÓČČ·“Ӧɜ³ÉĒāäåĖį£¬Š“³öĒāäåĖįÓėŅŅ“¼ŌŚ¼ÓČČŹ±·“Ó¦µÄ»Æѧ·½³ĢŹ½ ”£
£Ø2£©ŹŌ¹ÜIÖŠ·“Ó¦³żĮĖÉś³ÉäåŅŅĶ飬»¹æÉÄÜÉś³ÉµÄÓŠ»śĪļÓŠ _ ”¢
(Š“³öĮ½ÖÖÓŠ»śĪļµÄ½į¹¹¼ņŹ½)”£
£Ø3£©äåŅŅĶéµÄ·Šµć½ĻµĶ£¬Ņ×»Ó·¢£¬ĪŖĮĖŹ¹äåŅŅĶéĄäÄżŌŚŹŌ¹Ü¢ņÖŠ£¬¼õÉŁ»Ó·¢£¬ÉĻĶ¼ÖŠ²ÉČ”µÄ“ėŹ©ÓŠ ”¢ _”£
£Ø4£©ŌŚ½ųŠŠäåŅŅĶéÓėNaOHŅŅ“¼ČÜŅŗ¹²ČȵĊŌÖŹŹµŃ鏱£¬°ŃÉś³ÉµÄĘųĢåĶعżĻĀĶ¼ĖłŹ¾µÄ×°ÖĆ”£ÓĆĻĀĶ¼×°ÖĆ½ųŠŠŹµŃéµÄÄæµÄŹĒ _£»ĻĀĶ¼ÖŠÓŅ±ßŹŌ¹ÜÖŠµÄĻÖĻóŹĒ £»Ė®µÄ×÷ÓĆŹĒ ”£
¢ÅHBr + C2H5OH C2H5Br + H2O ””¢ĘCH2£½CH2 CH3CH2OCH2CH3
C2H5Br + H2O ””¢ĘCH2£½CH2 CH3CH2OCH2CH3
¢ĒŹŌ¹Ü¢ņČūÉĻ“ųÓŠµ¼¹ÜµÄČū×Ó²¢ŌŚĘäÖŠ¼ÓĖ®£»°ŃŹŌ¹Ü¢ņ·ÅČėŹ¢ÓŠĄäĖ®µÄÉÕ±ÖŠ£»Ź¹ÓĆ³¤µ¼¹ÜµČ
¢ĒŃé֤ɜ³ÉµÄĘųĢåŹĒŅŅĻ©£Ø»ņŃéÖ¤äåŅŅĶéÓėNaOH·¢ÉśĻūČ„·“Ó¦µÄ²śĪļ£©
¢ČøßĆĢĖį¼ŲČÜŅŗµÄ×ĻŗģÉ«ĶŹČ„ ””³żČ„ĘųĢåÖŠ»ģÓŠµÄÉŁĮæŅŅ“¼µČŌÓÖŹ
½āĪöŹŌĢā·ÖĪö£ŗ
¢ÅŌŚ¼ÓČȵÄĢõ¼žĻĀ£¬ŅŅ“¼ŗĶäå»ÆĒā·¢ÉśČ”“ś·“Ó¦£¬Éś³ÉäåŅŅĶéŗĶĖ®£¬Ōņ·“Ó¦µÄ»Æѧ·½³ĢŹ½ŹĒHBr + C2H5OH C2H5Br + H2O”£
C2H5Br + H2Oӣ
¢ĘÓÉÓŚŌŚÅØĮņĖįµÄ×÷ÓĆĻĀ£¬ŅŅ“¼Ņ²æÉÄÜ·¢ÉśĻūČ„·“Ӧɜ³ÉŅŅĻ©£¬Ņ²æÉÄÜ·¢Éś·Ö×Ó¼äµÄĶŃĖ®£¬Éś³ÉŅŅĆŃ£¬Ęä½į¹¹¼ņŹ½·Ö±šŹĒCH2£½CH2”¢CH3CH2OCH2CH3”£
¢ĒÓÉÓŚäåŅŅĶéŅ×»Ó·¢£¬ŌņŅŖŹ¹äåŅŅĶéĶéĄäÄżŌŚŹŌ¹Ü¢ņÖŠ£¬¼õÉŁ»Ó·¢£¬ĘäÕżČ·µÄ·½·ØÓŠŹŌ¹Ü¢ņČūÉĻ“ųÓŠµ¼¹ÜµÄČū×Ó²¢ŌŚĘäÖŠ¼ÓĖ®£»°ŃŹŌ¹Ü¢ņ·ÅČėŹ¢ÓŠĄäĖ®µÄÉÕ±ÖŠ£»Ź¹ÓĆ³¤µ¼¹ÜµČ”£
¢ČŌŚĒāŃõ»ÆÄʵē¼ČÜŅŗÖŠ£¬äåŅŅĶé·¢ÉśĻūČ„·“Ӧɜ³ÉŅŅĻ©£¬ŅŅĻ©ŗ¬ÓŠĢ¼Ģ¼Ė«¼ü£¬ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£¬ĖłŅŌøł¾Ż×°ÖĆĶ¼æÉÖŖ£¬øĆŹµŃéÄæµÄŹĒŃé֤ɜ³ÉµÄĘųĢåŹĒŅŅĻ©£Ø»ņŃéÖ¤äåŅŅĶéÓėNaOH·¢ÉśĻūČ„·“Ó¦µÄ²śĪļ£©£¬¶ųŹµŃéĻÖĻóŹĒøßĆĢĖį¼ŲČÜŅŗµÄ×ĻŗģÉ«ĶŹČ„”£µ«ÓÉÓŚŅŅ“¼Ņ×»Ó·¢£¬ĒŅŅŅ“¼Ņ²ÄÜŹ¹ĖįŠŌøßĆĢĖį¼ŲČÜŅŗĶŹÉ«£¬ĖłŅŌĖ®µÄ×÷ÓĆŹĒ³żČ„ĘųĢåÖŠ»ģÓŠµÄÉŁĮæŅŅ“¼µČŌÓÖŹ£¬·ĄÖ¹øÉČÅŅŅĻ©µÄŠŌÖŹ¼ģŃ锣
æ¼µć£ŗæ¼²éŅŅ“¼”¢äåŅŅĶ锢ŅŅĻ©µČÓŠ»śĪļµÄŠŌÖŹ”£


 Ņ»±¾ŗĆĢāæŚĖćĢāæØĻµĮŠ“š°ø
Ņ»±¾ŗĆĢāæŚĖćĢāæØĻµĮŠ“š°ø
| Äź¼¶ | øßÖŠæĪ³Ģ | Äź¼¶ | ³õÖŠæĪ³Ģ |
| øßŅ» | øßŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” | ³õŅ» | ³õŅ»Ćā·ŃæĪ³ĢĶĘ¼ö£” |
| ø߶ž | ø߶žĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õ¶ž | ³õ¶žĆā·ŃæĪ³ĢĶĘ¼ö£” |
| øßČż | øßČżĆā·ŃæĪ³ĢĶĘ¼ö£” | ³õČż | ³õČżĆā·ŃæĪ³ĢĶĘ¼ö£” |
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ĢĒĄą”¢ÓĶÖ¬”¢µ°°×ÖŹĪŖ¶ÆĪļŠŌŗĶÖ²ĪļŠŌŹ³ĪļÖŠµÄ»ł±¾ÓŖŃųĪļÖŹ”£
(1)µ°°×ÖŹ”¢µķ·Ū”¢Ö¬·¾ČżÖÖÓŖŃųĪļÖŹÖŠĖ®½āµÄ×īÖÕ²śĪļÄÜÓėŠĀÖĘCu(OH)2Šü×ĒŅŗ·“Ó¦µÄŹĒ________£¬ÖĘŌģ·ŹŌķµÄÖ÷ŅŖŌĮĻŹĒ________”£
(2)µ°°×ÖŹĖ®½āµÄ×īÖÕ²śĪļŹĒ____________”£
(3)ĻĀĮŠÓŠ¹ŲĖµ·ØÖŠÕżČ·µÄŹĒ____________”£
A£®µ°°×ÖŹÖŠÖ»ŗ¬C”¢H”¢OČżÖÖŌŖĖŲ
B£®ÓĶÖ¬ŌŚČĖĢåÖŠ·¢ÉśĖ®½āµÄ²śĪļŹĒ°±»łĖį
C£®ĢĒĄą²¢²»¶¼ÓŠĢšĪ¶
D£®ĢĒĄą”¢ÓĶÖ¬”¢µ°°×ÖŹ¶¼ÄÜ·¢ÉśĖ®½ā·“Ó¦
(4)µķ·ŪČÜŅŗŗĶµ°°×ÖŹČÜŅŗ¶¼ŹĒ½ŗĢ壬ÓĆŅ»Źų¹āĶعżĘäČÜŅŗ£¬¶¼²śÉś____________£¬Čō¼ģŃéĖüĆĒæÉŃ”ÓƵďŌ¼ĮŹĒ____________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĢīæÕĢā
ijČĖÉč¼Ęµķ·ŪĄūÓĆ·½°øČēĻĀĶ¼ĖłŹ¾£ŗ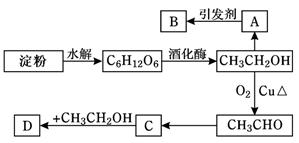
ĘäÖŠ£ŗAŹĒŅŅĻ©ÄܓߏģĖ®¹ū£¬BŹĒøß·Ö×Ó»ÆŗĻĪļ£¬DŹĒÓŠĖ®¹ūĻćĪ¶µÄĪļÖŹ”£Ēė»Ų“šŅŌĻĀĪŹĢā£ŗ
(1)”°C6H12O6”±µÄĆū³ĘŹĒ________£¬AµÄµē×ÓŹ½ĪŖ__________£¬CÖŠŗ¬ÓŠ¹ŁÄÜĶÅĆū³Ę £»
(2) A”śB·“Ó¦ĄąŠĶ_________________£»C”śD·“Ó¦ĄąŠĶ_________________
(3)Š“³öĻĀĮŠ×Ŗ»ÆµÄ»Æѧ·½³ĢŹ½
¢ŁA”śB£ŗ £¬
¢ŚC”śD£ŗ £¬
¢ŪCH3CH2OH”śCH3CHO£ŗ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ŅŅ“¼ĘūÓĶ×÷ĪŖŅ»ÖÖŠĀŠĶĒå½ąČ¼ĮĻ£¬ŹĒÄæĒ°ŹĄ½ēÉĻæÉŌŁÉśÄÜŌ“µÄ·¢Õ¹ÖŲµć£¬¶ųĒŅ¾ßÓŠ½ĻŗĆµÄ¾¼ĆŠ§ŅęŗĶÉē»įŠ§Ņę£¬ÖÕ½«³ÉĪŖĘūÓĶŗĶ²ńÓĶµÄĢꓜʷ”£
(1)Š“³öŅŅ“¼ĶźČ«Č¼ÉյĻÆѧ·½³ĢŹ½£ŗ______________________________”£
(2)ŅŅ“¼Č¼ÉÕŹ±Čē¹ūŃõĘų²»×ć£¬æÉÄÜ»¹ÓŠCOÉś³É”£ÓĆČēĶ¼×°ÖĆŃéÖ¤ŅŅ“¼µÄČ¼ÉÕ²śĪļÖŠÓŠCO”¢CO2”¢H2O£¬Ó¦½«ŅŅ“¼µÄČ¼ÉÕ²śĪļŅĄ“ĪĶعż(°“ĘųĮ÷“Ó×óµ½ÓŅµÄĖ³ŠņĢī×°ÖƱąŗÅ)________”£
(3)ŹµŃ鏱æɹŪ²ģµ½×°ÖĆ¢ŚÖŠAĘæµÄŹÆ»ŅĖ®±ä»ė×Ē”£AĘæČÜŅŗµÄ×÷ÓĆŹĒ________£»BĘæČÜŅŗµÄ×÷ÓĆŹĒ________£»CĘæČÜŅŗµÄ×÷ÓĆŹĒ_________________________”£
(4)×°ÖĆ¢ŪµÄ×÷ÓĆŹĒ________£»×°ÖĆ¢ŁÖŠĖłŹ¢µÄŹĒ________£¬×÷ÓĆŹĒ______________________”£
(5)×°ÖĆ¢ÜÖŠĖłŹ¢µÄ¹ĢĢåŅ©Ę·ŹĒ________£¬ĖüæÉŅŌŃéÖ¤µÄ²śĪļŹĒ________”£
(6)Ī²ĘųÓ¦ČēŗĪ“¦Ąķ£æ_________”£
(7)ŗ£µ×ÓŠ“óĮæµÄ¼×ĶéĖ®ŗĻĪļ”£µČÖŹĮæµÄ¼×ĶéŗĶŅŅ“¼ĶźČ«Č¼ÉÕ²śÉśĪĀŹŅĘųĢåCO2½Ļ¶ąµÄŹĒ________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗĪŹ“šĢā
ŹµŃéŹŅÖĘČ”ŅŅĖį¶”õ„µÄŹµŃé×°ÖĆÓŠČēÓŅĻĀĶ¼ĖłŹ¾Į½ÖÖ×°ÖĆ¹©Ń”ÓĆ”£ĘäÓŠ¹ŲĪļÖŹµÄĪļĄķŠŌÖŹ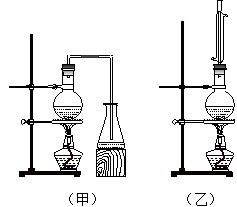
ČēĻĀ±ķ£ŗ
| | ŅŅĖį | 1-¶”“¼ | ŅŅĖį¶”õ„ |
| ČŪµć(”ę) | 16.6 | £89.5 | £73.5 |
| ·Šµć(”ę) | 117.9 | 117 | 126.3 |
| ĆܶČ(g/cm3) | 1.05 | 0.81 | 0.88 |
| Ė®ČÜŠŌ | »„ČÜ | æÉČÜ(9g/100gĖ®) | Ī¢ČÜ |
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø10·Ö£©Ä³»Æѧ»ī¶ÆŠ”×éÉč¼ĘŅŌĻĀ×°ÖĆ½ųŠŠ²»Ķ¬µÄŹµŃ锣ĘäÖŠaĪŖÓĆÓŚ¹ÄČėæÕĘųµÄĘųÄŅ£¬bĪŖĀŻŠż×“ĶĖ棬cÖŠŹ¢ÓŠ±łĖ®”£
£Ø1£©ČōÓĆA×°ÖĆ×öŅŅ“¼ÓėŅŅĖįµÄõ„»Æ·“Ó¦ŹµŃ飬Ōņ»¹ŠčĮ¬½ÓµÄ×°ÖĆŹĒ £ØĢīŠņŗÅ£©£¬øĆ×°ÖĆÖŠÓ¦¼ÓČėŹŌ¼Į ”£“ÓŹµŃé°²Č«½Ē¶Čæ¼ĀĒ£¬A×°ÖĆŹŌ¹ÜÖŠ³ż¼ÓČė·“Ó¦ŅŗĶā£¬»¹Šč¼ÓČėµÄ¹ĢĢåĪļÖŹŹĒ ”£AÖŠ·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ£ŗ ”£
£Ø2£©øĆŠ”×éĶ¬Ń§Óū×öŅŅ“¼Ńõ»Æ³ÉŅŅČ©µÄŹµŃé²¢ŹÕ¼ÆÉś³ÉµÄ²śĪļ£¬ŌņӦєÓƵÄ×°ÖĆŹĒ £ØĢīŠņŗÅ£©£¬·“Ó¦µÄ»Æѧ·½³ĢŹ½ĪŖ______________________________________ ”£
£Ø3£©ČōŌŁÓĆÖʵƵÄŅŅČ©ČÜŅŗ½ųŠŠŅų¾µ·“Ó¦£¬Ń”ÓĆ2%µÄAgNO3ČÜŅŗŗĶ2%µÄĻ”°±Ė®ÅäÖĘŅų°±ČÜŅŗµÄ²Ł×÷ŹĒ ”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
£Ø 11·Ö£©ŅŅ“¼µÄ·ŠµćŹĒ78”ę£¬ÄÜÓėĖ®ŅŌČĪŅā±Č»ģČÜ”£ŅŅĆѵķŠµćĪŖ34.6”ę£¬ÄŃČÜÓŚĖ®£¬ŌŚ±„ŗĶNa2CO3ČÜŅŗÖŠ¼øŗõ²»ČÜ£¬ŅŅĆŃ¼«Ņ×Č¼ÉÕ”£ŹµŃéŹŅÖĘĆѵķ“Ó¦ŌĄķŹĒ£ŗ
2CH3CH2OH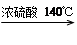 H2O + CH3CH2”ŖO”ŖCH2CH3 (ŅŅĆŃ)
H2O + CH3CH2”ŖO”ŖCH2CH3 (ŅŅĆŃ)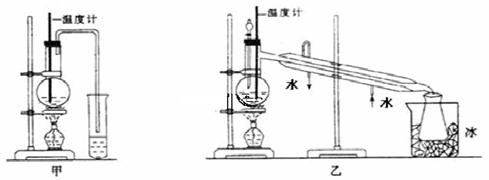
£Ø1£©¼×Ķ¼ŗĶŅŅĶ¼ŹĒĮ½Ģ׏µŃéŹŅÖĘŅŅĆѵÄ×°ÖĆ£¬Ń”×°ÖĆ___(Ģī”°¼×”±»ņ”°ŅŅ”±)×īŗĻĄķ£¬ĄķÓÉŹĒ_ ”£
£Ø2£©·“Ó¦ŅŗÖŠÓ¦¼ÓČė·ŠŹÆ£¬Ęä×÷ÓĆŹĒ____________”£
£Ø3£©·“Ó¦ÖŠĪĀ¶Č¼ĘµÄÕżČ·Ī»ÖĆŹĒĖ®ŅųĒņÖĆÓŚ________________________”£
£Ø4£©ÓĆÉĻŹö×°ÖĆŅŅÖʵƵÄŅŅĆŃÖŠæÉÄÜŗ¬ÓŠ“óĮæµÄŌÓÖŹ£¬øĆŌÓÖŹŹĒ__________£¬³żČ„ÕāÖÖŌÓÖŹµÄ¼ņŅ×·½·ØŹĒ________________________________”£
£Ø5£©Čē¹ūĪĀ¶ČĢ«øߣØČē170”ę£©£¬½«»į·¢ÉśŅ»øöÓŠ»śø±·“Ó¦£¬·“Ó¦·½³ĢŹ½ĪŖ£ŗ__________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗŹµŃéĢā
ĪŖĮĖ²ā¶ØŅŅ“¼µÄ½į¹¹Ź½ŹĒ £¬ĄūÓĆŅŅ“¼ŗĶÄʵķ“Ó¦£¬Éč¼ĘČēĶ¼×°ÖĆ½ųŠŠŹµŃ飬ŌŚÉÕĘæÖŠ·ÅČė×ćĮæµÄÄĘ£¬“Ó·ÖŅŗĀ©¶·ÖŠ»ŗ»ŗµĪČėŅ»¶ØĮæµÄŅŅ“¼£¬Ķعż²āĮæĮæĶ²ÖŠĖ®µÄĢå»ż£¬¾ĶæÉÖŖ·“Ӧɜ³ÉµÄĒāĘųµÄĢå»ż”£
£¬ĄūÓĆŅŅ“¼ŗĶÄʵķ“Ó¦£¬Éč¼ĘČēĶ¼×°ÖĆ½ųŠŠŹµŃ飬ŌŚÉÕĘæÖŠ·ÅČė×ćĮæµÄÄĘ£¬“Ó·ÖŅŗĀ©¶·ÖŠ»ŗ»ŗµĪČėŅ»¶ØĮæµÄŅŅ“¼£¬Ķعż²āĮæĮæĶ²ÖŠĖ®µÄĢå»ż£¬¾ĶæÉÖŖ·“Ӧɜ³ÉµÄĒāĘųµÄĢå»ż”£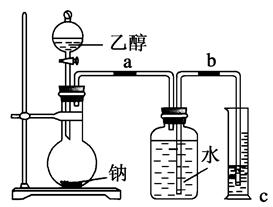
£Ø1£©ŹµŃéĒ°¼ģŃéøĆ×°ÖƵÄĘųĆÜŠŌµÄŹµŃé²Ł×÷ŹĒ
__________________________________________________ӣ
£Ø2£©ÓŠČĖČĻĪŖ×°ÖĆÖŠÓŠæÕĘų£¬Ėł²āµÄĘųĢåĢå»żÓ¦æŪ³ż×°ÖĆÖŠæÕĘųµÄĢå»ż£¬²ÅŹĒĒāĘųµÄĢå»ż£¬ÄćČĻĪŖ________£ØĢī”°ÕżČ·”±»ņ”°²»ÕżČ·”±£©”£
£Ø3£©Čē¹ūŹµŃéæŖŹ¼Ē°bµ¼¹ÜÄŚĪ“³äĀśĖ®£¬ŌņŹµŃé½į¹ū½«________£ØĢī”°Ę«“ó”±»ņ”°Ę«Š””±£©”£
£Ø4£©Čō²āµĆÓŠ1.15 g C2H6O²Ī¼Ó·“Ó¦£¬°ŃĮæĶ²cÖŠµÄĖ®µÄĢå»ż»»Ėć³É±ź×¼×“æöĻĀH2µÄĢå»żĪŖ280 mL£¬ŹŌ½įŗĻ¼ĘĖćŗĶĢÖĀŪ£¬ÅŠ¶ĻĻĀĆę£Ø¢ń£©ŗĶ£Ø¢ņ£©Į½Ź½ÖŠ£¬ÄÄøöÕżČ·________”£
²éæ““š°øŗĶ½āĪö>>
æĘÄæ£ŗøßÖŠ»Æѧ Ą“Ō“£ŗ ĢāŠĶ£ŗµ„Ń”Ģā
ĻĀĮŠ·“Ó¦ÖŠ£¬ŹōÓŚ¼Ó³É·“Ó¦µÄŹĒ
| A£®¼×ĶéÓėĀČĘų·“Ó¦ÖĘČ”Ņ»ĀČ¼×Ķé | B£®ŅŅĖįÓėŅŅ“¼·“Ó¦ÖĘČ”ŅŅĖįŅŅõ„ |
| C£®±½·ÓÓė±„ŗĶäåĖ®·“Ӧɜ³ÉČżäå±½·Ó | D£®ŅŅĻ©ÓėĀČ»ÆĒā·“Ó¦ÖĘČ”ĀČŅŅĶé |
²éæ““š°øŗĶ½āĪö>>
°Ł¶ČÖĀŠÅ - Į·Ļ°²įĮŠ±ķ - ŹŌĢāĮŠ±ķ
ŗž±±Ź”»„ĮŖĶųĪ„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±ØĘ½ĢØ | ĶųÉĻÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | µēŠÅÕ©Ę¾Ł±Ø×ØĒų | É꥜Ź·ŠéĪŽÖ÷ŅåÓŠŗ¦ŠÅĻ¢¾Ł±Ø×ØĒų | ÉęĘóĒÖČؾŁ±Ø×ØĒų
Ī„·ØŗĶ²»Į¼ŠÅĻ¢¾Ł±Øµē»°£ŗ027-86699610 ¾Ł±ØÓŹĻä£ŗ58377363@163.com