
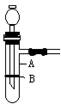
 ���������շ�������
���������շ������� ������ʱͬ����ϰ��ϵ�д�
������ʱͬ����ϰ��ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
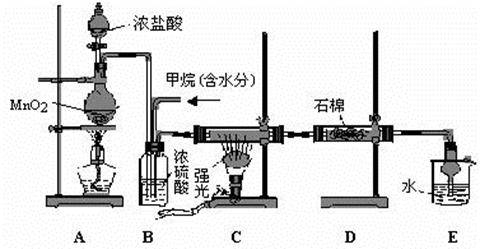
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ������
 ���л��ܼ���������ˮ���ܶ�1.46��1.52g/cm3���ж�������ʱ����Fe2(CO)9��60�淢����ȼ�����������Ϳ������������ȡ����ʻ��������Ʊ�ԭ�����£�
���л��ܼ���������ˮ���ܶ�1.46��1.52g/cm3���ж�������ʱ����Fe2(CO)9��60�淢����ȼ�����������Ϳ������������ȡ����ʻ��������Ʊ�ԭ�����£�
| A������������Ӧԭ�����Ʊ��ߴ��� |
| B���Ʊ�Fe(CO)5Ӧ�ڸ��������������½��� |
C����ӦFe(s)+5CO(g) Fe(CO)5(g)��ƽ�ⳣ������ʽΪ Fe(CO)5(g)��ƽ�ⳣ������ʽΪ |
| D��Fe(CO)5Ӧ�ܷ⡢�������ܹⲢ����������ˮҺ�����档 |
 ��Һ11.72g��ȫȼ�գ��õ�30.536gCO2��5.4gH2O��1.6g����ɫ��ĩ��
��Һ11.72g��ȫȼ�գ��õ�30.536gCO2��5.4gH2O��1.6g����ɫ��ĩ�� ��
���鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����
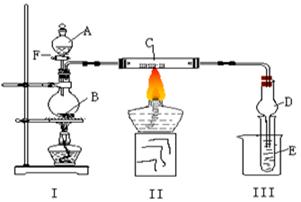
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ�ʵ����

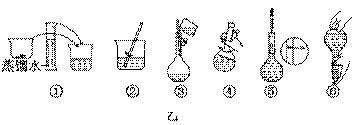
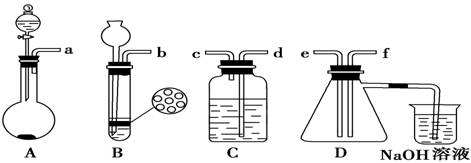
 NaOH����ʱ�����ڸ�����ѡȡ����������С��������������ĸ����������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ����������������ĸ��.
NaOH����ʱ�����ڸ�����ѡȡ����������С��������������ĸ����������ͼ��ѡ������ȷ��ʾ����λ�õ�ѡ����������������ĸ��.| | a | b | c | d | e |
| �����С/g | 100 | 50 | 20 | 10 | 5 |
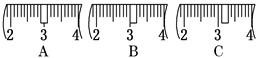
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
| A��ʳ�� | B������FeCl3��Һ | C������ | D���� |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ������ ���ͣ���ѡ��
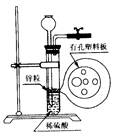
| A���٢ڢ� | B���ڢ� |
| C���ڢۢ� | D���٢ۢ� |
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com