
��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϡ��о���ѧϰС���ͬѧ��Ϊ�ⶨij��ͬƷ����þ�Ͻ𣨲�������Ԫ�أ���þ����������������������ֲ�ͬʵ�鷽������̽������д���пհס�
[̽��һ] ʵ�鷽������þ�Ͻ�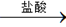 �ⶨ������������
�ⶨ������������
ʵ��װ������ͼ���������ۣ� 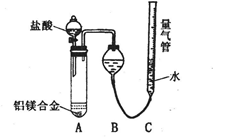
��1����Ӧ���,ÿ���1���Ӷ�ȡ������������������С��ֱ�����䡣���������С��ԭ����
��������ʵ�������Ӱ�����س��⣩��
��2��Ϊʹ�ⶨ��������ܾ�ȷ��ʵ����Ӧע���������˼��װ�õ������ԡ�������������ʹ�Ͻ���ȫ�ܽ�Ͱ���1�������⣬����д�����㣺
��
��
��3������÷�����ƽȷ��ȡ0.51gþ���Ͻ���ʵ�飬��������������Ϊ560 mL��������ɱ����������������Ͻ���þ����������������д��������̣�___________ ____________________________________________________________________________��
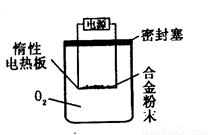
[̽����] ʵ�鷽��������Bg��һƷ����þ�Ͻ��ĩ����������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������ա� 
�������ۣ�
��1��������Mg��������������ʵ���л���ⶨ�������� ��
��2���ñ���������ʵ��ʱ��װ��������Ҫ����O2�����ʵ��� mol���ú�B�����ʽ��ʾ����
[ʵ����չ] �о�С���ij��ɫ������Һ����ʵ�飬���ָ���Һ������Ӧʱ�ų�H2�����ж��������ӣ�Mg2+��Cu2+��Ba2+��H+��Ag+��SO42����HCO3����OH����NO3�������������������,���ܴ����ڴ���Һ�е��ǣ�
�ٵ�������Ӧ������Al3+ʱ��ԭ��Һ���ܴ������ڵ����� ��
�ڵ�������Ӧ������[Al(OH)4]��ʱ��ԭ��Һ���ܴ������ڵ������� ��
(12�֣� [̽��һ]��1����Ӧ���ȣ������¶Ƚϸߣ��¶Ƚ���ʱ�����������С��1��
��2���ٵ���������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ���ڶ���ʱ��֤�����밼Һ�����͵���ƽ��(��1��)��3��47��1% (4��)
[̽����]��1�����պ��������� (1��) ��2��B/36 (2��)
[ʵ����չ]��Mg2+��SO42����H+ ��Ba2+��OH-��NO3��(��1��)
�������������[̽��һ]��1�����ý�������ķ�Ӧ�Ƿ��ȷ�Ӧ�������������С��ԭ���Ƿ�Ӧ���ȣ������¶Ƚϸߣ��¶Ƚ���ʱ�����������С��
��2����Ҫʹ�����е�ѹǿ��Ȳ��ܼ���ȷ��������������C�ĸ߶ȣ�ʹC��Һ����BҺ����ƽ�����ų���Ϊ��ԭ������ʱ��֤�����밼Һ�����͵���ƽ��
��3��560 mL��0.025mol��Mg~~~H2����Al~~~3/2 H2����
24n(Mg)+27n(Al)=0.51��n(Mg) +3/2 n(Al)=0.025�����n(Al)=0.01mol��n(Mg)=0.01mol��
m(Mg)= 24n(Mg)=0.024(g)���Ͻ���þ����������Ϊ0.024g/0.51g*100%=47.1%��
[̽����]��1��������þ��������������ʵ���л���ⶨ��һ�������ǣ���ȫ��Ӧ�����ɵĹ����������
��2������Ͻ�ȫ�������������������ٵģ���ϵʽΪAl~~~3/4O2,�������װ��������Ҫ����O2�����ʵ���ΪB/36mol��
[ʵ����չ] (1) ����Al3+��֤����ϵ�д���H+
����H+��ͬ���ڵ���Ba2+��Cu2+��Mg2+��H+��SO42-��NO3-��Cl-
��Һ��ɫ���ų�Cu2+
������NO3-������Al��Ӧ����H+������NO����NO2���ʶ�NO3-������
���µ�Ba2+��SO42-����ͬʱ����
�ʶ���ϵ�д���Ba2+��Mg2+��H+��Cl-����Mg2+��H+��Cl-��SO42-�������������Ag+�����ܴ��ڣ�Ag2SO4��AgCl���ܽ�Ȳ���
(2) ����AlO2-��֤����ϵ��OH-
2Al + 2OH- + 2H2O ="===" 2AlO2- + 3H2��
����OH-�����ܺ���Mg2+������Mg(OH)2��������������H+��������Ag+��������Cu2+��������HCO3-
ʣ��ΪBa2+��SO42-��SO32-��NO3-��OH-��Cl-
��Һ�б��������ӣ�Ba2+
����û��SO32-��SO42-
ֻʣ�£�Ba2+��NO3-��OH-��Cl-
���㣺̽�����ʵ���ɻ�������ʵĺ��� þ�Ļ�ѧ���� ���Ļ�ѧ����
��������Ҫ�������ʺ����IJⶨ����ʵ��ԭ����װ�õ����⡢ʵ�鷽����Ƶȣ��Ѷ��еȣ�����ʵ��ԭ���ǽ���Ĺؼ����Ƕ�֪ʶ���ۺϿ��飬��Ҫѧ������֪ʶ�Ļ������ۺ�����֪ʶ�������⡢��������������



| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| ����������Һ |
| ���� |

�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ��Ķ�����
| NaOH��Һ |
| ���� |

 �������ۣ�
�������ۣ��鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
| NaOH��Һ |
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2011-2012ѧ���Ϻ���������ѧ�߶���ѧ�����п��Ի�ѧ�Ծ� ���ͣ�ʵ����
��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϡ��о���ѧϰС�����λͬѧ��Ϊ�ⶨij��þ3��һ5������þ�Ͻ�(��������Ԫ��)��þ����������������������ֲ�ͬʵ�鷽������̽������д���пհס�
��̽��һ��ʵ�鷽������þ�Ͻ� �ⶨʣ�����������
�ⶨʣ�����������
�� �⣺ʵ���з�����Ӧ�Ļ�ѧ����ʽ��
ʵ�鲽�裺
(1)��ȡ5.4g��þ�Ͻ��ĩ��Ʒ��Ͷ��VmL 2��0mol��L-1NaOH��Һ�У���ַ�Ӧ��NaOH��Һ�����V��
(2)���ˡ�ϴ�ӡ�����������塣�ò�������δϴ�ӹ��壬���þ������������ (�ƫ�ߡ���ƫ�͡�)��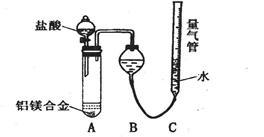
��̽������ʵ�鷽������þ�Ͻ� �ⶨ������������ʵ��װ�á�
�ⶨ������������ʵ��װ�á�
�������ۣ�
(1)ijͬѧ�����ʵ��װ�ò������ƣ�Ӧ��A��B֮������һ�������������װ�á��������ǣ� (���Ҫ������Ҫ��)��
(2)Ϊʹ�ⶨ��������ܾ�ȷ��ʵ����Ӧע���������(д������)��
��
�� 
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2015�츣��ʡ��һ��ѧ�����п��Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��þ�Ͻ��ѳ�Ϊ�ִ����졢������������ҵ����Ҫ���ϡ��о���ѧϰС���ͬѧ��Ϊ�ⶨij��ͬƷ����þ�Ͻ𣨲�������Ԫ�أ���þ����������������������ֲ�ͬʵ�鷽������̽������д���пհס�
[̽��һ] ʵ�鷽������þ�Ͻ� �ⶨ������������
�ⶨ������������
ʵ��װ������ͼ���������ۣ�

��1����Ӧ���,ÿ���1���Ӷ�ȡ������������������С��ֱ�����䡣���������С��ԭ����
��������ʵ�������Ӱ�����س��⣩��
��2��Ϊʹ�ⶨ��������ܾ�ȷ��ʵ����Ӧע���������˼��װ�õ������ԡ�������������ʹ�Ͻ���ȫ�ܽ�Ͱ���1�������⣬����д�����㣺
��
��
��3������÷�����ƽȷ��ȡ0.51gþ���Ͻ���ʵ�飬��������������Ϊ560 mL��������ɱ����������������Ͻ���þ����������������д��������̣�___________ ____________________________________________________________________________��
[̽����] ʵ�鷽��������Bg��һƷ����þ�Ͻ��ĩ����������ͼ��ʾװ�õĶ��Ե��Ȱ��ϣ�ͨ��ʹ�������ա�
�������ۣ�
��1��������Mg��������������ʵ���л���ⶨ�������� ��
��2���ñ���������ʵ��ʱ��װ��������Ҫ����O2�����ʵ��� mol���ú�B�����ʽ��ʾ����
[ʵ����չ] �о�С���ij��ɫ������Һ����ʵ�飬���ָ���Һ������Ӧʱ�ų�H2�����ж��������ӣ�Mg2+��Cu2+��Ba2+��H+��Ag+��SO42����HCO3����OH����NO3�������������������,���ܴ����ڴ���Һ�е��ǣ�
�ٵ�������Ӧ������Al3+ʱ��ԭ��Һ���ܴ������ڵ����� ��
�ڵ�������Ӧ������[Al(OH)4]��ʱ��ԭ��Һ���ܴ������ڵ������� ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com