
(1)
(2)�����ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ��_____________����ԭ����__________________��
(3)������ö�Ԫ��������һ���ǻ�����±ԭ�ӣ����õ���±���ﶼֻ��һ�֣���д���ñ��Ͷ�Ԫ���Ľṹ��ʽ��
(1)0.125 0.3 0.1 5��12��4
(2)�� ��Ϊ��ʵ��ʽ����ԭ�Ӹ����Ѵﱥ��
(3)C(CH2OH)4
���������״̬��״��������ˮ����Һ̬�����е�Cԭ��ȼ������CO2����Ҫ��O2�����Ӧ�ø����ɵ�CO2�������ȣ���O2��CO2��������Ӧ������������٣�˵��
![]()
�豥�Ͷ�Ԫ���ķ���ʽΪCnH2n+2Ox������ȼ�շ���ʽΪ��
![]()
n n+1
0.125 n(H2O)
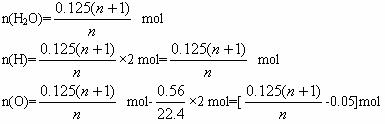
���ݶ�Ԫ����������
![]()
���n=5
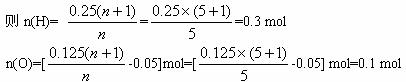
�������N(C)��N(H)��N(O)=0.125��0.3��0.1=5��12��4��ʵ��ʽΪC5H12O4������ʵ��ʽ����ԭ�Ӹ����Ѵﵽ���ͣ��ʷ���ʽΪC5H12O4��


 ���ĺ����Ͼ�������ϵ�д�
���ĺ����Ͼ�������ϵ�д�
| �꼶 | ���пγ� | �꼶 | ���пγ� |
| ��һ | ��һ��ѿγ��Ƽ��� | ��һ | ��һ��ѿγ��Ƽ��� |
| �߶� | �߶���ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
| ���� | ������ѿγ��Ƽ��� | ���� | ������ѿγ��Ƽ��� |
��Ŀ�����л�ѧ ��Դ������������ ���ͣ�022
����(1)3.4 g����C��H��O�����ʵ����ֱ�Ϊ��
����n(C)��______ mol��n(H)��______ mol��n(O)��______ mol���ô���C��H��Oԭ����֮��Ϊ��__________��
����(2)�����ϱ�ֵ�ܷ�ȷ���ô��ķ���ʽ����ԭ����_______________________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
����һЩֻ��C��H��O����Ԫ�ص��л������ȼ��ʱ���ĵ����������ɵĶ�����̼���������3��4��
��1����Щ�л����У���Է���������С�Ļ�������________��
��2��ij����̼ԭ������ͬ�������л�������ǵ���Է��������ֱ�Ϊa��b��a��b������a-b�ض���________������һ�����֣�����������
��3������Щ�л�������һ�ֻ�����������������ǻ���ȡ0.2625 g���л���ǡ���ܸ�25.00 mL 0.100 mol��L-1 NaOH��Һ��ȫ�кͣ��ɴ˿��Լ����֪�û��������Է���������__________________�������Ƶ����ķ���ʽӦΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ�� ���ͣ�
������CO��HCOOH��HOOC��CHO����ȩ�ᣩ�ֱ�ȼ��ʱ�����ĵ����������ɵĶ�����̼������ȶ���1��2�������ߵķ���ʽ���Էֱ��ǣ�CO����H2O���ͣ�CO��2��H2O����Ҳ����˵��ֻҪ����ʽ���ϣ�CO��n(H2O)m�� n��m��Ϊ���������ĸ����л������ȼ��ʱ���ĵ����������ɵĶ�����̼�����������1��2��
����һЩֻ��C��H��O����Ԫ�ص��л������ȼ��ʱ���ĵ����������ɵĶ�����̼���������3��4��
��1����Щ�л����У���Է���������С�Ļ�������________��
��2��ij����̼ԭ������ͬ�������л�������ǵ���Է��������ֱ�Ϊa��b��a��b������a-b�ض���________������һ�����֣�����������
��3������Щ�л�������һ�ֻ�����������������ǻ���ȡ0.2625 g���л���ǡ���ܸ�25.00 mL 0.100 mol��L-1 NaOH��Һ��ȫ�кͣ��ɴ˿��Լ����֪�û��������Է���������__________________�������Ƶ����ķ���ʽӦΪ____________________��
�鿴�𰸺ͽ���>>
��Ŀ�����л�ѧ ��Դ��2012-2013ѧ���Ϻ����ɽ���������ѧ����ĩ���Ի�ѧ�Ծ��������棩 ���ͣ�ʵ����
��˾ƥ�ֵ���Ч�ɷ�������ˮ���ᣨ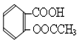 ����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳ�����ˮ���ᣬ�Ʊ�����Ҫ��ӦΪ��
����ʵ������ˮ���ᣨ���ǻ������ᣩ�������[(CH3CO)2O]Ϊ��Ҫԭ�Ϻϳ�����ˮ���ᣬ�Ʊ�����Ҫ��ӦΪ��
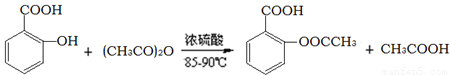
�����������£�
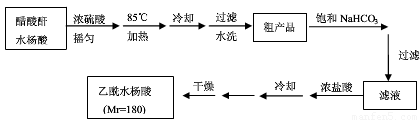
��֪��ˮ���������ˮ���������ˮ����������������ˮ����������ˮ�ֽ����ɴ��ᡣ
�ش��������⣺
��1���ϳɹ���������ʵļ��ȷ����� ��
��2���Ʊ������У�ˮ������γɾۺ���ĸ����д���þۺ���Ľṹ��ʽ ��
��3���ֲ�Ʒ�ᴿ��
�� ��������������NaHCO3�ܽ�ֲ�Ʒ��Ŀ���� ���жϸù��̽����ķ����� ��
�� ��Һ��������Ũ�����У������������� ��
�� �������ղ�Ʒ���Ƿ���ˮ����Ļ�ѧ������ ��
��4����˾ƥ��ҩƬ������ˮ���Ậ���IJⶨ���裨�ٶ�ֻ������ˮ������ϣ����ϲ����뷴Ӧ����
��.��ȡ��˾ƥ����Ʒm g����.����Ʒ���飬����V1 mL a mol��L-1NaOH�������������ȣ���ȥ���ϵȲ������������Һ������ƿ����.����ƿ�еμӼ��μ��ȣ���Ũ��Ϊb mol��L-1�ı����ᵽ�ζ�ʣ���NaOH��������������ΪV2mL��
�� д������ˮ���������NaOH��Һ���ȷ�����Ӧ�Ļ�ѧ����ʽ
��
�� ��˾ƥ��ҩƬ������ˮ�������������ı���ʽΪ ��
�鿴�𰸺ͽ���>>
����ʡ������Υ���Ͳ�����Ϣ�ٱ�ƽ̨ | �����к���Ϣ�ٱ�ר�� | ����թƭ�ٱ�ר�� | ����ʷ���������к���Ϣ�ٱ�ר�� | ������Ȩ�ٱ�ר��
Υ���Ͳ�����Ϣ�ٱ��绰��027-86699610 �ٱ����䣺58377363@163.com